Recording and interpretation of ocular movements: saccades, smooth pursuit, and optokinetic nystagmus
Article information
Abstract
The ultimate role of ocular movements is to keep the image of an object within the fovea and thereby prevent image slippage on the retina. Accurate evaluations of eye movements provide very useful information for understanding the functions of the oculomotor system and determining abnormalities therein. Such evaluations also play an important role in enabling accurate diagnoses by identifying the location of lesions and discriminating from other diseases. There are various types of ocular movements, and this article focuses on saccades, fast eye movements, smooth pursuit, and slow eye movements, which are the most important types of eye movements used in evaluations performed in clinical practice.
INTRODUCTION
Visual acuity is best when the image of an object is accurately focused on the fovea. This requires the image of the object to be formed within 0.5º of the fovea and the speed of image movement on the retina to be maintained within 5º/s. Visual acuity generally begins to decrease when the speed at which an object’s image moves on the retina (retinal slip) exceeds 2º/s. Vision becomes blurred if the image of the object moves away from the fovea or it is formed at a location other than the central concave. Images formed on the periphery of the retina must be moved to the fovea (gaze shift), and those formed on the fovea should be kept stable so as not to appear shaking (gaze holding).1 Saccades are eye movements made to rapidly move the gaze from one object to another, which is the most important function when performing numerous daily activities such as visual searching, reading a book, or reading a computer monitor.1 Additionally, slow eye movements also play the significant role in maintaining the gaze, and these types of eye movements include smooth pursuit, vestibulo-ocular reflex (VOR), optokinetic nystagmus, and vergence (convergence and divergence).1
INSPECTION METHOD
The methods used to record and interpret ocular movements with video-oculography (VOG) are described below. When performing VOG, drugs that may affect test results (e.g., tranquilizers or vestibular depressants) should be stopped 24-48 hours beforehand, and the patient’s medical records should be checked for cognitive dysfunction, visual impairment, and ptosis. It is also necessary to determine whether the assessment might be impeded by comorbidities (e.g., cognitive dysfunction, visual impairment, or ptosis) or medications (e.g., tranquilizers or vestibular depressants). The patient wearing VOG goggles sits upright on an examination chair and looks at a target 1 m away. The inspection should be performed in a dark room. Calibration needs to be performed individually for each patient to ensure accurate evaluations. The examiner asks the patient to keep their eyes on the target for a set period of time while the eye movements are recorded.
SACCADES
Saccades are responsible for rapidly moving the gaze from one fixation point to another, but involuntary eye movements may also occur that cause the gaze object to move away from the fovea. Volitional and reflexive saccades can be evaluated by examining eye movements, and saccadic intrusion and saccadic oscillation can occur involuntarily in pathological conditions.2 This phenomenon can be caused by lesions in various structures such as the cerebellum, brainstem, basal ganglia, and cerebral hemispheres, and it prevents the gaze from staying in one place.
Saccade recording methods
Saccades are recorded with the patient seated in an examination chair and wearing goggles. The pupils must be clearly visible via the examination monitor. The patient is asked to fixate their gaze on the target point that is about 1 m away, and the lights in the examination room are turned off. The eye position is then calibrated by instructing the patient to follow the target point as rapidly as possible using only ocular motion; that is, while keeping the head motionless. The target point is set to move from the center of the patient to the left and the right within a range of 10-15º. The examiner must ensure that the patient’s head does not move during the examination.
Vertical saccades can also be examined by setting the target points to be positioned up and down in addition to horizontal saccades with the target points located to the left and right along the light bar. In addition to measuring fixed saccades with the lights alternately appearing at fixed points, random saccades also can be evaluated by making irregular changes to the duration and location of the light on the left and right sides. The inspection usually takes 60-90 seconds, but for some purposes it may take longer than 3 minutes. It is also possible to perform either a binocular or monocular examination. Memory-guided saccades are measured by first moving the target point with the patient gazing in a fixed direction, and then asking them to move their eyes based on what they have remembered. Also, antisaccades are measured by asking the patient to look in the direction opposite to the direction in which the light moves.
In addition to evaluating random saccades, the saccadic system can be evaluated through optokinetic nystagmus (OKN) or caloric-induced nystagmus. It can also be evaluated via the occurrence of corrective rapid saccades after head-impulse tests (HITs). OKN and caloric-induced nystagmus assess whether nystagmus occurs as a result of corrective saccades according to ocular deviation.3 In cases assessed using HIT, suspicion may arise when there is an abnormality in the VOR but no corrective saccades occur.4
Interpretation of volitional saccades
Latency
Latency is the time taken from the appearance of the target to the occurrence of saccades, and is normally 200-250 ms. The latency increases with age, and it may show abnormalities in the presence of various brain diseases, but the clinical significance of such latency changes is unclear.5
Accuracy
Even in a person with normal vision, a saccade to a target that is more than 15º away usually does not reach the target, and small saccades will follow it. Hypometria refers to the saccades requiring three or more movements to reach the target, or the gain being lower than the normal value. Conversely, hypermetria refers to the saccade overshooting the target, which is usually due to malfunction of the cerebellum.6 In addition, hypometric and hypermetric saccades can occur in patients with ocular myasthenia gravis, which may reflect a central mechanism of compensation for gaze-holding deficits.
Velocity
The velocity of saccades increases logarithmically with their amplitude. In other words, since the normal range of velocity varies with the amplitude, judging the velocity decrease of a saccade requires judging whether there is an outlier that is based on the amplitude (Figs. 1, 2). Saccades are slower when a lesion invades burst cells in the brainstem.7
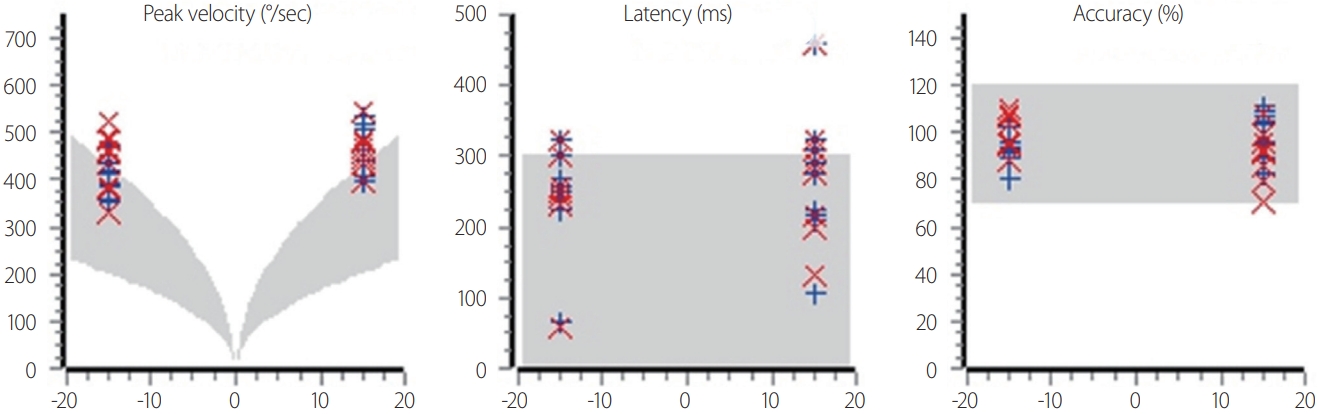
Analysis of fixed saccades. The maximum velocity, latency, and accuracy are measured. The gray areas indicate the normal values.
Saccadic intrusion and saccadic oscillation
Intermittent abnormal rapid eye movements are called saccadic intrusion, while the continuous repetition of such movements is called saccadic oscillation.8 Saccadic oscillation should be distinguished from nystagmus, which begins with slow eye movements and progresses to rapid eye movements that move objects away from the fovea. Saccadic oscillation can be subdivided according to the direction of rapid eye movements and the interval between them. The test method for detecting saccadic intrusion and saccadic oscillation is the same as that used for saccades. A light is presented at the front of the test patient to confirm that there is no saccadic intrusion or saccadic oscillation other than nystagmus (with gaze fixation). Similarly, new spontaneous eye movement or any changes in the existing saccadic intrusion or saccadic oscillation (without gaze fixation) are checked for after removing the visual target.
Interpretation of saccadic intrusion and saccadic oscillation
According to the presence or absence of intersaccadic latency between waveforms and repetitive saccades, saccadic intrusion and saccadic oscillation are classified as described below (Fig. 3).

Saccadic intrusion and saccadic oscillation. (A) Square-wave jerks (SWJ), macro-square-wave jerks (MSWJ), saccadic pulse (SP), and double saccadic pulse (DSP). (B) Square wave oscillation (SWO). (C) Macrosaccadic oscillation (MSO). (D) Saccadic pulse trains (SPT).
Square-wave and macro-square-wave jerks
Square-wave jerks and macro-square-wave jerks (the latter is where the amplitude exceeds 5º) have both been reported to occur frequently in neurodegenerative diseases such as progressive supranuclear palsy, but they can occur frequently even in normal old age, which restricts their diagnostic value in clinical practice.9
Saccadic pulse
A saccadic pulse is an eye movement that deviates from the gaze point and then returns to the gaze point through glissadic drift. The continuous appearance of these saccadic pulses can be confused with nystagmus (especially decelerating nystagmus), and they are classified according to whether the eye movements deviating from the gaze point are saccades or glissadic drift.10
Macrosaccadic oscillation
Macrosaccadic oscillation refers to an involuntary saccade that passes the gaze point continuously left and right or up and down with a certain intersaccadic interval. Macrosaccadic oscillation is caused by continuous fluctuations around the gaze point due to hypermetric saccades, and so spontaneous saccades often accompany saccadic hypermetria.11
Ocular flutter and opsoclonus
Ocular flutter is a series of saccades that occur consecutively within a single plane and without an intersaccadic interval. This condition can also occur while at rest, but is often caused by eye movement or blinking. Unlike ocular flutter, opsoclonus occurs continuously without intersaccadic intervals while having horizontal, vertical, and torsional components, and the saccades are not restricted to a single plane. These characteristics have resulted in it also being called ‘dancing eyes’ and ‘saccadomania'. Both ocular flutter and opsoclonus are pathological phenomena and can occur in cerebellar disorders.12
SMOOTH PURSUIT AND OKN
Functions and physiology of smooth pursuit
The purpose of smooth pursuit is to keep the image of a slowly moving object stable within the fovea. Smooth pursuit is also involved in the initial acceleration that occurs in OKN, and it also plays a role in suppressing or reinforcing the VOR during visual stimulation. Therefore, smooth pursuit, optokinetic reflex, and visual control of the VOR usually show the same degree of impairment.13
Smooth pursuit is induced by retinal slip on the retina. When an object moves within the fovea, smooth pursuit starts after a latency of 100-125 ms. The velocity of the object and the velocity of the eyeball approximately coincide at 100-300 ms after the start of the movement due to the acceleration during pursuit initiation.14 Once the eye movement has stopped accelerating, the velocity of smooth pursuit is kept constant in accordance with the velocity of the object during the pursuit maintenance period.15 Unlike the initiation of smooth pursuit that occurs due to retinal slip, the maintenance of smooth pursuit is thought to be regulated by extraretinal signals such as efference copy, in which the brain copies and memorizes the eye movement command and then monitors the eye movement performed based on it.16
Physiology of OKN
The optokinetic system is a phylogenetically old system.17 While smooth pursuit is involved in maintaining the gaze of a moving object within the fovea, the optokinetic system is a primitive type of smooth pursuit involving the entire retina. The exact anatomical pathway of the optokinetic system is not known, but it is thought to overlap with that underlying smooth pursuit, and it extends from the visual association area (Brodmann areas 18 and 19) to the abducens nucleus in the pons, which is the center of horizontal eye movements.18 Visual information is transmitted to the oculomotor cells via two pathways: 1) the direct pathway with characteristic rapid movement transmission and 2) the indirect pathway shared with the VOR.19 Indirect pathway play an important role in the velocity storage mechanism that is gradually reinforced by OKN, and optokinetic afternystagmus (OKAN) occurs once the stimulation has finished. The direct pathway is to the oculomotor cells via the primary visual cortex (also called the cortical pathway) and the indirect pathway is via the pretectal nuclei and the accessory optic tract (also called the subcortical pathway). While smooth pursuit activates only the direct pathway, the optokinetic system activates both the direct and indirect pathways. The direct pathway is mainly involved in the early stages of OKN and OKAN.20
Recording methods for smooth pursuit and OKN
When examining smooth pursuit, the test patient is asked to follow a small target that is moving up, down, left, and right at 1 m or more from the head in an upright posture. These movements are first performed at a low speed (10-20º/s), and the occurrence of catch-up saccades and back-up saccades is checked for. A cylinder with stripes (an OKN drum) is useful for inducing slight asymmetry or reversal of smooth pursuit. When there is a disorder of smooth pursuit on one side, it can be observed that corrective nystagmus decreases when the OKN drum is turned toward that side.
Smooth pursuit is affected by the patient’s concentration during the examination and any drugs being taken. The characteristics of smooth pursuit also vary with age. Smooth pursuit is not well developed in infants, and it may appear to varying degrees in children. In the elderly, the function of smooth pursuit worsens with increasing age, and so the age of the patient should be taken into account when interpreting the results. In normal subjects, smooth pursuit in the horizontal direction is symmetric, whereas the gain in vertical pursuit is smaller for downward than upward motion, and so catch-up saccades can be observed.
Examinations of smooth pursuit can apply different types of stimulation according to their purpose. In order to evaluate the initial induction phase of smooth pursuit, the patient should look at a target moving at a constant speed (ramp stimulus) or at a target moving at a constant speed after an instantaneous movement (step-ramp stimulus). On the other hand, evaluations of the maintenance phase of smooth pursuit should involve the patient looking at a target moving at a constant speed (triangle stimulus) or following a smoothly oscillating trajectory (sinusoidal stimulus) (Fig. 4).

Types of stimulation for analyzing smooth pursuit: (A) step-ramp, (B) sinusoidal, and (C) triangle stimuli.
The target must occupy the entire field of view in examinations of OKN in order to identify differences from the findings of smooth-pursuit tests. In general, an OKN drum is rotated at various speeds or the stripes are displayed on the wall inside the drum and rotated for inspection. OKAN is evaluated by recording the nystagmus remaining after applying stimulation in a dark room. The smooth-pursuit system mainly works from when the stripe movement begins until the eye movement reaches the maximum velocity, after which the optokinetic system mainly acts to maintain the maximum velocity. Rotating the OKN drum downward to induce OKN upward may be helpful for observing convergence-retraction nystagmus.
Interpretation of smooth pursuit and OKN
Smooth pursuit
Sinusoidal stimuli
Sinusoidal stimuli can be used to evaluate the maintenance of smooth pursuit, and its parameters include gain, phase, and asymmetry (Fig. 5).
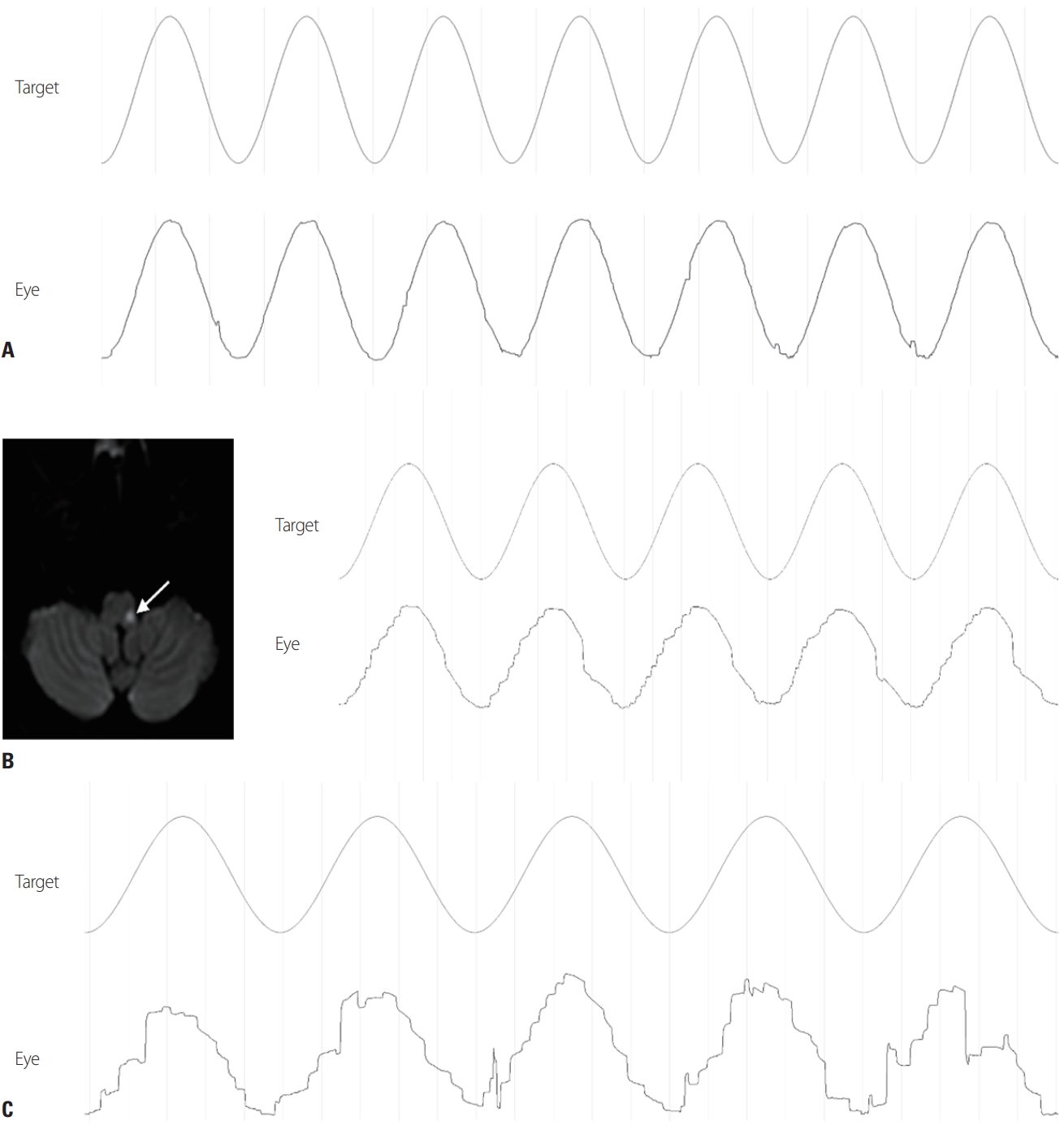
(A) Horizontal smooth pursuit in a normal subject. (B) Contralateral impairment of smooth pursuit in a patient with lateral medullary infarction (arrow). (C) Bilateral smooth-pursuit impairment in a patient with spinocerebellar ataxia.
Gain
The gain refers to the ratio of the eye velocity to the target velocity, and is affected by the size, brightness, acceleration, velocity, and trajectory of the target. In general, the gain is maintained well up to an object velocity of 100º/s but decreases rapidly for higher velocities.21 The gain also decreases gradually with increasing age.22,23 When the target oscillates sinusoidally, its peak velocity is determined by the amplitude (A) and frequency (f) according to Vpeak=2πAf. Therefore, the examiner can combine various amplitudes and frequencies to apply specific stimulation conditions. In general, the maximum velocity of the target is set within 50º/s, and the oscillation frequency is set within 2 Hz. Even at the same maximum speed, smooth pursuit is affected by acceleration, so gains should not be compared simply according to the maximum velocity of the target.
Asymmetry
Smooth pursuit in the horizontal direction is symmetrical, but for vertical motion it is common for the gain to be smaller for downward smooth pursuit than for upward motion. Disorders of smooth pursuit can be divided into four types: unilateral (appears only in one direction regardless of the location on the retina), retinal (occurs in both directions on only one side of the visual field), craniotopic (occurs only on one side based on the position of the head), and omnidirectional (disorders in all directions) (Table 1).
Unidirectional pursuit paresis occurs in the presence of unilateral lesions in the angular gyrus and the temporo-occipito-parietal junction in the direction of the lesion.24 This region corresponds to the middle temporal and medial superior temporal (MT/MST) areas in monkeys, and is involved in motion perception.25 Lesions in these areas are usually accompanied by contralateral hemianopia, but smooth-pursuit disorders occur irrespective of visual field defects and do not occur in hemianopia caused by lesions before the lateral geniculate body or by lesions confined to the cortex.26 Paresis on the contralateral side of the lesion is mild and is mainly observed in acute lesions in the nondominant hemisphere. The frontal eye field receives information from the MT/MST areas, and unilateral lesions in the frontal eye field and posterior frontal lobes also cause smooth-pursuit paralysis toward the lesion.27 Even if the smooth-pursuit pathway from the cerebrum to the dorsolateral pontine nucleus (DLPN) is damaged, paralysis can occur toward the lesion, and it can also occur in the posterior thalamus, internal capsule posterior limb, pretectum, and tegmentum regions.28 DLPN lesions can impair smooth pursuit to the ipsilateral side, with abnormalities observed during both initiation and maintenance.29 In lateral medullary syndrome, impaired smooth pursuit toward the opposite side of the lesion is observed. In patients with nystagmus due to a lesion of the peripheral vestibular system, a smooth-pursuit disorder can appear toward the opposite side of the lesion. This is because the slow phase of nystagmus affects smooth pursuit toward the lesion, which is not a true disorder of smooth pursuit. Therefore, in patients with spontaneous nystagmus, attention should be paid to how smooth pursuit is interpreted, and the slow phase of nystagmus should be considered when examining asymmetry.
Omnidirectional pursuit paresis is caused by diffuse lesions in the cerebrum, cerebellum, or brainstem, various drugs, fatigue, and aging (Table 2). Paralysis in all directions has no particular localizing value, but it is a sensitive indicator of brain dysfunction. Anticonvulsants, narcotics, tranquilizers, and decreased alertness may cause abnormal findings.
Phase
Phase is used to quantify the temporal coincidence between the stimulus and eyeball movement, and there is almost complete temporal coincidence without any phase lag in ideal smooth pursuit. On the other hand, with an increase in stimulus acceleration, a phase lag is commonly noted.
Step-ramp stimuli
The initiation phase of smooth pursuit is evaluated by measuring the position, velocity, and acceleration of the eyeball when applying a step-ramp or ramp stimulus. The evaluation parameters include the onset latency, initial acceleration, and directional deficit.
Onset latency
When applying a ramp stimulus there is latency of about 100 ms between stimulus initiation and eye movement. However, in a step-ramp stimulus the target momentarily steps in the opposite direction prior to applying the ramp, and the latency increases to about 150 ms.30
Initial acceleration
The initial acceleration occurs for 40 ms after the onset of smooth pursuit, and is unaffected by the velocity, brightness, and position of the stimulus in the visual field. The initial acceleration ranges from 40º/s to 100º/s, and it varies between individual subjects. The initial acceleration is higher in the vertical direction than in the horizontal direction. The initial acceleration in the horizontal direction is increased when the target moves toward the center of the visual field, and in the vertical direction it is increased upon stimulation of the lower visual field.31
Optokinetic nystagmus
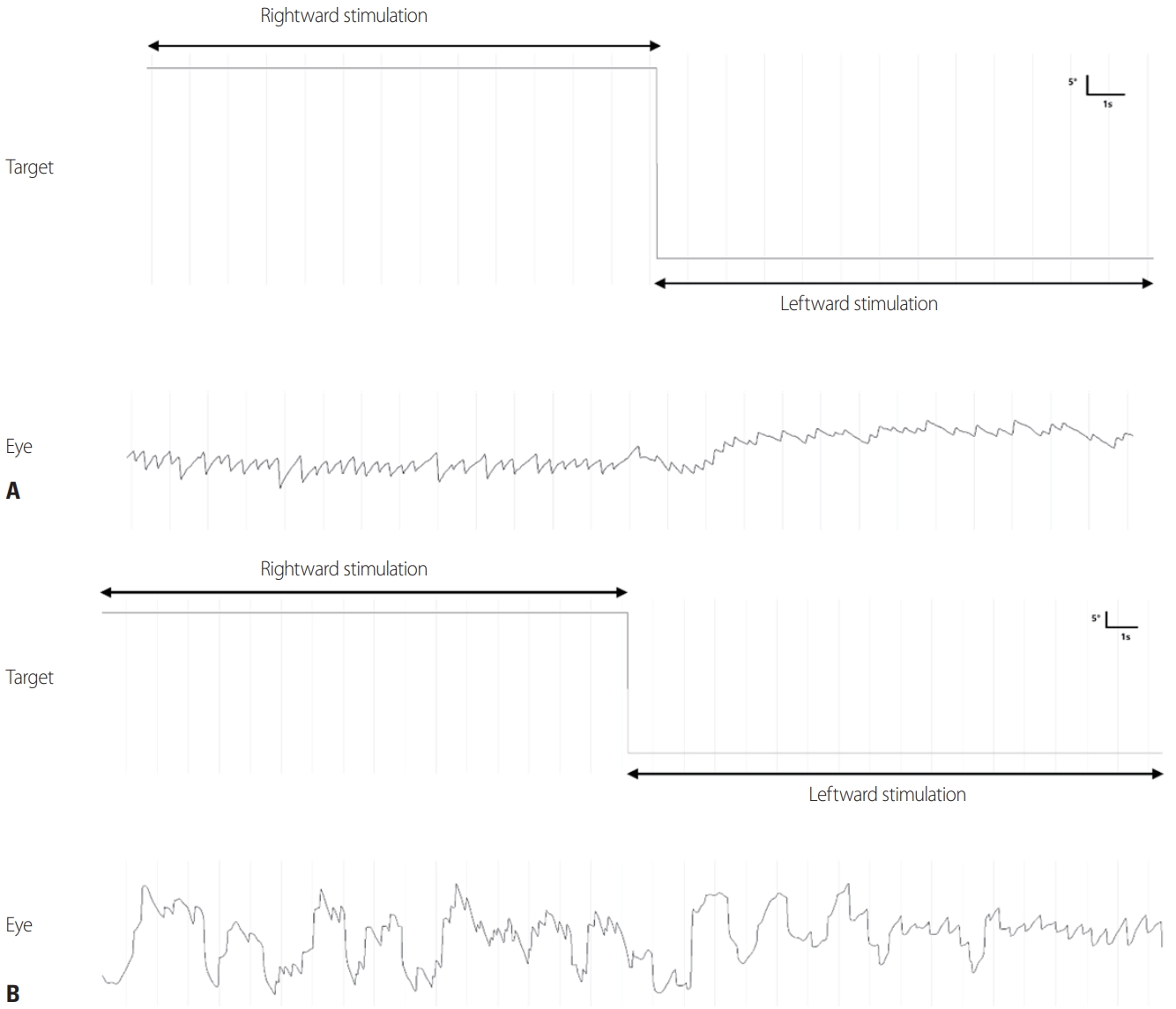
The slow component of OKN generally appears as an abnormality in smooth pursuit, and the fast component is observed along with an abnormality in saccadic eye movements. Interpreting OKN is very similar to interpreting smooth pursuit. Abnormalities in the optokinetic system can be observed in the presence of peripheral and central vestibular lesions, as well as lesions in the visual pathway.32 In unilateral lesions in the peripheral vestibular system, the slow-phase velocity of OKN toward the lesion increases and decreases in opposite directions, so an asymmetry is observed in bidirectional OKN.33 Also, OKAN decreases in both directions in unilateral lesions, and it is more severe when the stimulus is toward the direction opposite to the lesion.34 In bilateral labyrinth lesions, OKN is normal but OKAN disappears.35,36 OKN is directed to the left when the OKN drum is turned to the right in a normal subject (Fig. 6A), whereas OKN reversal is characteristically observed in the rotational direction in cases of congenital nystagmus (Fig. 6B). OKAN may disappear if the velocity storage mechanism is impaired by lesions in the central vestibular system. Any abnormality in the velocity, accuracy, or latency of saccadic eye movements may result in abnormal OKN. Nystagmus that persists in the same direction after stopping stimulation in the dark is called OKAN, while nystagmus that lasts for 10-30 seconds and then changes direction is called secondary OKAN.
CONCLUSION
Eye-movement tests provide valuable data for understanding brain function and providing clues to disease localization. The parameters to be measured in saccades are velocity, latency, accuracy, and the presence of saccadic intrusion and saccadic oscillation. Saccadic disorders can be classified according to whether the saccadic pulse, the saccadic step, or the correspondence between the saccadic pulse and saccadic step is inappropriate, and they are commonly observed in cerebellar diseases and neurodegenerative disorders. Smooth pursuit can be quantified by measuring its gain, phase, and asymmetry. Smooth-pursuit eye movements involve diverse anatomical structures and complex interactions, and so abnormalities therein may be caused by various lesions including those in the cerebrum, cerebellum, and brainstem.
Notes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. 2022R1A2B5B01001933) and by the Fund of the Biomedical Research Institute, Jeonbuk National University Hospital.