Application of respiratory function tests in patients with neurological diseases
Article information
Abstract
Breathing is controlled by complex interactions between the central and peripheral nervous systems in conjunction with the respiratory system. Neurological diseases predispose patients to nocturnal desaturation and pneumonia due to respiratory dysfunction, which increases mortality, daytime sleepiness and fatigue, and reduces the quality of life. Respiratory function tests are required to identify respiratory function decline and to consider compensatory management. This review summarizes the characteristics of several respiratory function tests and their applications to neurological diseases.
INTRODUCTION
Respiration is controlled by the cerebral cortex and respiratory center that is constituted by neuron networks in the pons and medulla.1,2 Moreover, breathing is resulting from the contraction of inspiratory and expiratory muscles under the control of respiratory neurons in the brainstem and the ventral horn of the upper cervical spinal cord.3 Respiratory distress conditions such as dyspnea, orthopnea, and central and obstructive sleep apnea can be attributed to diseases that impair the functions of respiratory neurons, the respiratory center, and inspiratory and expiratory muscles.1,2,4-11 Neuromuscular diseases, stroke, Parkinson’s disease, and multiple system atrophy predispose patients to dysfunction of either the respiratory neuron or respiratory muscles and manifests alongside nocturnal hypoventilation and pneumonia, thereby shortening the survival duration of patients and reducing their quality of life.5-11 Respiratory function tests should be considered for identifying respiratory dysfunction and determining whether assisted devices are required for patients with neurological diseases that involve the respiratory system. Herein we review the characteristics and clinical applications of respiratory function tests.
RESPIRATORY FUNCTION TESTS
Spirometry
Spirometry is performed to differentiate between airway obstruction and restrictive pulmonary disease in order to assess whether the patient has a chronic obstructive disease (e.g., asthma) and to evaluate a patient with dyspnea or wheezing.12 The test involves repeatedly inhaling for as long as possible and exhaling for 6-15 seconds with maximal effort at least three times, with the obtained results interpreted using the following three variables: vital capacity (VC), forced expiratory volume in 1 second (FEV1), and FEV1/VC% (i.e., FEV1/VC×100). For reproducibility, tests are repeated until the difference between the maximum values of forced VC (FVC) and FEV1 is within 5% or 0.1 L.
A decreased FEV1 /VC% indicates airway obstruction, whereas a decreased total lung capacity and a normal FEV1/VC% indicates restrictive pulmonary disease.12 Restrictive patterns may not be fully revealed through spirometry alone when there is moderate-to-severe airway obstruction. The VC is divided into the following two types according to the test procedure: (1) FVC involves the patient inhaling maximally and exhaling as fast as possible in a sitting or supine position, whereas (2) slow VC involves the patient inhaling maximally and exhaling slowly. FVC is usually measured in a sitting position, but occasionally in the supine position. Spirometry it easy to perform, but it has the disadvantage of requiring the orofacial muscle to be strong enough to hold the mouthpiece during the test.13,14
Blood gas measurements
Blood gas measurements include arterial and venous blood gas analyses such as pulse oximetry, end-tidal oxygen (O2) and end-tidal carbon dioxide (CO2) measurements, and transcutaneous CO2 measurement. These measure O2 saturation, O2, and CO2 concentrations in exhaled air, and the concentration of blood gas through the skin, respectively. These measurements have the advantage of noninvasive monitoring of hypercapnia and hypoxemia in the presence of respiratory dysfunction.
Arterial blood gas analyses are performed to determine the oxygenation, ventilation, acid-base status, and O2-carrying capacity of the subject, which are measured using the O2 pressure and oxyhemoglobin saturation; CO2 pressure; pH; and partial pressure of oxygen (PaO2), oxyhemoglobin saturation, total hemoglobin, and dyshemoglobin saturation; respectively.15 They are sometimes performed to confirm the therapeutic effect of mechanical ventilation or O2 supplementation or to determine intubation timing. Arterial blood is often sampled, but venous blood may be used instead to evaluate goal-directed therapy for septic shock, or where perform an arterial puncture is difficult in patients with acute illness.16,17 Results may be imprecise if the storage time is prolonged at room temperature, and so the test should be performed within 30 minutes of blood collection. In blood samples from patients with remarkably increased leukocytes, PaO2 could decrease rapidly due to O2 consumption by leukocytes (i.e., leukocyte larceny) after blood collection.18-20 The sample may also be contaminated by the anticoagulant, air, or saline, leading to erroneous results.15
Pulse oximetry has some limitations. It can only detect hypoxemia early and cannot detect hypercapnia. When a patient presents with apnea, there might be a delay before this is reflected in the results due to significant O2 reserves in the blood.21
End-tidal CO2 measurement is useful when there is no abnormality in O2 saturation and only in hypercapnia due to a high O2 concentration in inhaled air. The results of end-tidal CO2 measurement are sometimes not strongly correlated with the actual arterial blood concentration due to alterations by various physiological and pathological conditions such as cyanotic heart disease, airway obstruction, mouth breathing, and O2 supplementation.22 The results obtained using a divided nasal cannula have the advantage of a smaller difference in blood CO2 concentration than for those obtained using a face mask. However, a method that uses a divided nasal cannula cannot detect air that is breathed through the mouth, and the tube can sometimes become clogged by water vapor or mucus.21,23
Impairment of alveolar ventilation results is relative small changes in the CO2 concentration due to CO2 buffering, whereas O2 concentration more accurately reflects the apnea in that condition. Moreover, if alveolar hypoventilation induces hypoxemia, O2 saturation is hardly altered due to considerable O2 reserves in the blood; instead, hypoxemia is reflected more promptly by end-tidal O2 concentration.21,24
Measuring the transcutaneous CO2 only roughly represents the arterial CO2 concentration and is affected by skin thickness, age, cardiac function, local metabolism, and peripheral perfusion.25,26
Peak cough expiratory flow measurement
Coughing plays an important role in airway protection by removing pathogens from and maintaining the airway, and involves the glottis first closing and the expiratory muscle contracting, and the glottis then reopens to exhale air and other substances from the airway. Peak cough expiratory flow is measured by blocking the nose with a clip and inhaling maximally to total lung capacity, and then coughing.10 A value higher than 400 L/min are considered normal and one lower than 270 L/min could indicate a risk of pneumonia.27
Measurements of maximal inspiratory pressure, maximal expiratory pressure, and sniff nasal inspiratory pressure
A decrease in VC indicates either restrictive pulmonary disease or inspiratory muscle weakness.28 Decreased FEV1 or FEV1/VC% indicates airway obstruction or expiratory muscle weakness. The maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and sniff nasal inspiratory pressure (SNIP) are often tested when the results of respiratory tests are abnormal in the absence of obstructive airway and respiratory pulmonary disease, or when the following diseases that involve respiratory muscles are suspected: respiratory muscle weakness associated with malnutrition, myasthenia gravis, Guillain-Barré syndrome, spinal cord injury, amyotrophic lateral sclerosis (ALS), stroke, and muscular dystrophy.11,29-31 These tests are often performed in a sitting position and have advantages of being relatively simple to measure and noninvasive (Fig. 1).28

Apparatus for measuring maximal inspiratory pressure, maximal expiratory pressure, and sniff nasal inspiratory pressure.
MIP is determined by the peak pressure while performing maximal and static inspiration (Muller’s maneuver) that starts from functional residual capacity after full expiration. MEP is determined by the peak pressure while performing maximal and static expiration (Valsalva maneuver) that starts from the total lung capacity after full inspiration. Both MIP and MEP are measured three times, and the largest value is selected. 11,28 Patients with generalized muscle weakness present with decreases in both MIP and MEP, whereas those with only diaphragm weakness only present with a decrease in MIP.
Sniff inspiratory pressure is determined by the inspiration pressure during the maximal sniff maneuver, which starts from the forced residual capacity after full expiration to minimize the residual volume and is divided into transesophageal, esophageal, and nasal pressures based on the measurement method. Among them, SNIP is widely used because it is less invasive. SNIP measures the peak inspiratory pressure five times with a nasal plug in one nostril and the other one occluded, and is determined as the highest value among them.11,14 SNIP measures global inspiratory muscle strength more accurately and has the advantage of less variability than MIP, whereas MIP measures diaphragm strength more specifically than does SNIP.11,32-35
MIP and MEP are difficult to measure when the patient has a significant orofacial weakness, and MIP, MEP, and SNIP are more difficult to perform when patients have cognitive impairment.
Transdiaphragmatic pressure measurement
The intrathoracic pressure decreases and the intra-abdominal pressure increases when the diaphragm contracts during respiration. The transdiaphragmatic pressure is calculated by subtracting the esophageal pressure measured using a catheter placed in the stomach from the gastric pressure measured using a catheter placed in the esophagus, and this indirectly indicates diaphragm strength. The transdiaphragmatic pressure is normally 10 cmH2O during small breaths and increases to 150 cmH2O during inspiration with maximal effort, which is less varied and a far higher value than the former.36
The transdiaphragmatic pressure can specifically reflect the diaphragm strength, but its measurement has some limitations: it is invasive, can be painful for the patient, is risky if a patient has dysphagia, and measurements are complicated. It may also be difficult to place the catheter into the stomach if the patient has severe respiratory muscle weakness.11
Phrenic nerve stimulation
The phrenic nerve that controls the diaphragm originate from the C3–C5 spinal roots and descend along the neck to allow direct stimulation to the neck. Phrenic nerve stimulation measures the diaphragmatic twitch magnitude using surface and reference electrodes placed in the seventh or eighth intercostal spaces and on the ipsilateral arm, respectively. It is performed using electrical or magnetic transcutaneous stimulation from the posterolateral side of the sternocleidomastoid muscle at the cricoid cartilage level.37-40 The normal value of the transdiaphragmatic pressure measured using phrenic nerve stimulation is 25-35 cmH2O.
Because this measurement method does not require patient effort, it is valuable for those with impaired consciousness or cognitive impairment, but it has the following limitations: first, the transdiaphragmatic pressure is affected by the impedances of the abdomen and rib cage, and increases when the abdominal fat is thick. Second, the transdiaphragmatic pressure could be altered by diaphragmatic contraction just before the measurement due to twitch potentiation.11 Third, it should be avoided in patients in the intensive care unit (ICU) with an external pacemaker, and should be carefully considered in those with an internal jugular catheter or cardiac pacemaker.40
Diaphragm imaging
Diaphragm abnormalities can be identified via chest X-rays, fluoroscopy, and ultrasonography. Chest X-rays can confirm abnormalities such as hemidiaphragm elevation, but they are mostly taken to confirm pulmonary disease rather than diaphragm strength.11
Fluoroscopy and ultrasonography are performed instead of chest X-rays to evaluate diaphragm strength. Fluoroscopy allows the real-time evaluation of diaphragm strength by measuring the excursion of the diaphragm dome during inhalation and exhalation with maximal effort, but it has the limitation that it exposes the patient to radiation.11 Ultrasonography also allows real-time measurements of the diaphragmatic thickness (which indirectly reflects diaphragm strength) at the end of expiration using a transducer placed in the midaxillary line at the intercostal space level between the seventh and ninth ribs.11 The diaphragm is a hypoechoic structure seen between the peritoneal and diaphragmatic pleurae that appear as hyperechoic lines, and it is normally thinner than 2 mm. Patients are considered to have diaphragmatic weakness if the diaphragm does not thicken during inspiration.11,41
Fluoroscopy and ultrasonography can be applied to patients in the ICU because they can be performed with a portable device, and have the advantages of being noninvasive and can be carried out without a mouthpiece, and hence are applicable to patients with significant orofacial weakness.11 However, both tests have limitations. The result may be a false negative if the breathing of a patient with neuromuscular disease is reliant on abdominal muscle contraction, or a false positive if the anterior diaphragm performs paradoxical cephalad movement during inspiration.11
IMPLICATIONS OF RESPIRATORY FUNCTION TESTS IN NEUROLOGICAL DISEASES
Respiration is regulated by the cerebral cortex and brainstem and occurs through contraction of the inspiratory and expiratory muscles that are innervated by the phrenic nerve, motor neurons, and phrenic nucleus in the upper cervical spinal cord; respiratory distress is therefore induced by abnormalities in one or more lesions in those areas.1-11 Based on these features, neurological diseases that exhibit respiratory dysfunction are categorized according to whether the lesion is in the brain, spinal cord, motor neuron, nerve, neuromuscular junction, or muscles.
Respiratory abnormalities in stroke differ depending on the stroke location. Cerebral hemispheric lesions manifest with decreased strengths of the chest wall and diaphragm on the side opposite the stroke, bilateral hemispheric lesions induce Cheyne-Stokes respiration or apnea due to periodic palsy of the vocal cord, and brainstem lesions induce central sleep apnea, obstructive sleep apnea, and alteration in respiration rhythm. Conditions such as spinal cord injury and postpolio syndrome manifest respiratory distress due to abnormalities in the spinal cord.9,42-46 Examples of diseases that involve motor neurons, nerves, neuromuscular junctions, and muscles include spinal muscular atrophy (SMA) types 1c, 2, and 3a, spinal and bulbar muscular atrophy, ALS, Guillain-Barré syndrome, critical-illness polyneuropathy, myasthenia gravis, Lambert-Eaton syndrome, botulism, organophosphate poisoning, Duchenne muscular dystrophy, myotonic dystrophy, Pompe disease, periodic paralysis, and inflammatory myopathy.47-59 Diseases such as myotonic dystrophy and Duchenne muscular dystrophy may also cause conditions associated with sleep-disordered breathing such as obstructive and central sleep apnea in addition to respiratory muscle weakness. Multiple sclerosis also manifests as respiration dysfunction due to certain causes such as respiratory muscle weakness, respiratory control impairment, sleep-disordered breathing, and neurogenic pulmonary edema.60 Parkinson’s disease and multiple-system atrophy present restrictive and peripheral obstructive patterns on spirometry, and respiratory muscle weakness induces decreases in MIP and MEP that are negatively correlated with the disease severity.9,42
Patients with respiratory dysfunction are predisposed to respiratory complications, which consequently lead to increased mortality, pneumonia, and reduced quality of life due to daytime sleepiness or fatigue. Respiratory function tests are therefore required to identify respiratory dysfunction before such conditions appear, and several assistant devices are used according to the obtained results. Respiratory function tests including MIP, MEP, phrenic nerve conduction study, nocturnal O2 saturation, and CO2 concentration are recommended for patients with stroke.61,62 Guidelines on the management of ALS, SMA, Guillain-Barré syndrome, and Pompe disease recommend that patients are monitored. 59,63-66 The values of MIP and SNIP are also related to the prognosis. When the MIP is reduced to less than 60 cmH2O, the survival duration and quality of life of a patient with ALS deteriorates, and when SNIP is reduced to lower than 40 cmH2O, patients with ALS are more likely to die within 6 months.14,67,68
Noninvasive ventilation is found to be assistant device to ameliorate respiratory distress and improve survival in neurological diseases such as ALS, and it can also be considered in conditions such as nocturnal hypoventilation associated with inspiratory muscle weakness based on the following criteria: the presence of orthopnea, MIP <40 cm-H2O or <60 cmH2O, SNIP <40 cmH2O, VC <50% predicted or 80%, daytime hypercapnia of partial pressure of carbon dioxide >45 mmHg, nocturnal hypoxemia, or symptomatic sleep-disordered breathing.63,64,69 A mechanical insufflation/exsufflation device can be considered for reducing the risk of pneumonia related to expiratory muscle weakness based on the following criteria: peak cough expiratory flow <160 L/min and MEP <60 cmH2O.21,69,70 Diaphragmatic strength can also be evaluated using slow VC or the difference between the FVC values measured in supine and sitting positions in situations where the above-mentioned tests for evaluating diaphragm strength are not available or where patients with neurological disease present with significant orofacial weakness.71-73
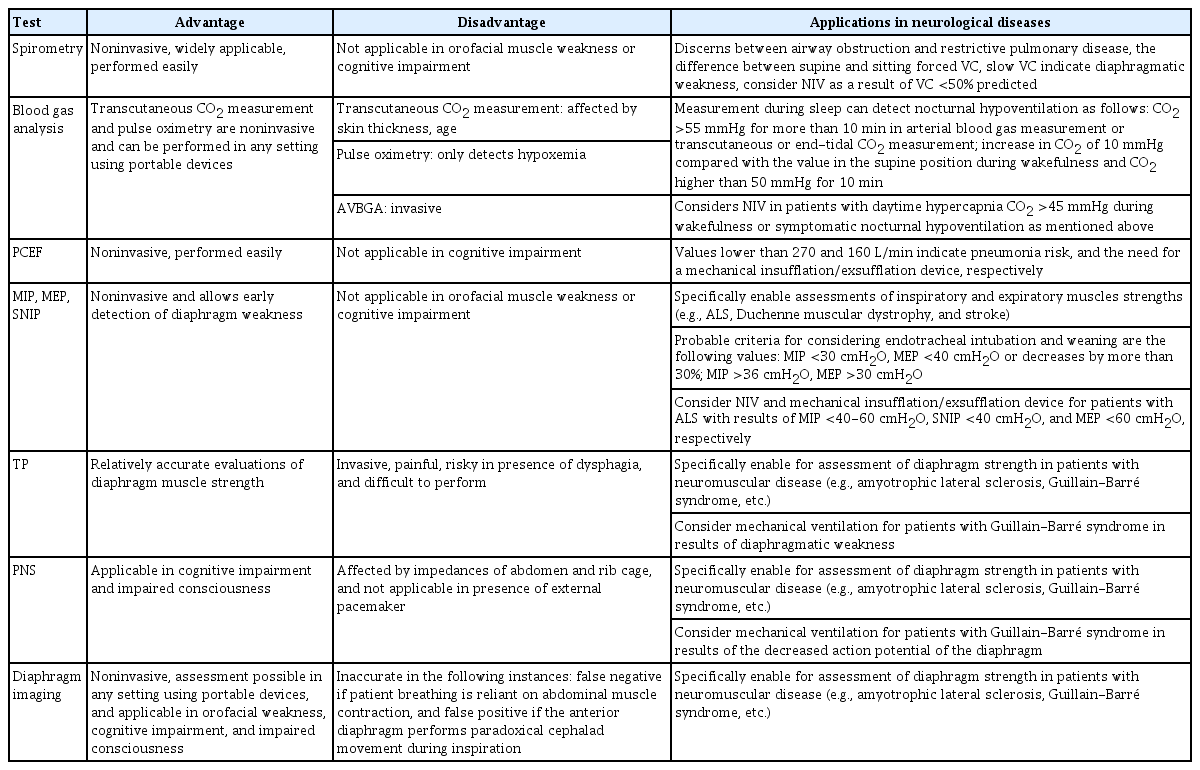
VC, MIP, and MEP can also be used to predict the need for endotracheal intubation and mechanical ventilation by applying the following criteria: VC, MIP, and MEP <20 mL/kg, 30 cmH2O, and 40 cmH2O, respectively, or decreases by more than 30%.74 Respiratory function tests are also considered for weaning off mechanical ventilation. MEP, MIP, and rapid shallow breathing index calculated as the tidal volume divided by the respiratory rate of higher than 30 cmH2O, higher than 36 cmH2O, and lower than 105 breaths/min/L, respectively, probably indicate successful weaning.75-77 The characteristics and clinical applications of respiratory function tests are summarized in Table 1.
CONCLUSION
In various neurological diseases, respiratory dysfunction caused by various conditions such as central and obstructive sleep apnea, inspiratory and expiratory muscle weakness, and lung volume loss may lead to increases in the risks of mortality and pneumonia. Respiratory function tests should therefore be considered at an appropriate time to detect respiratory dysfunction and to allow for the application of auxiliary devices.
Notes
Conflicts of Interest
The authors declare no conflicts of interest.