Exercise-induced rhabdomyolysis with acute kidney injury complicated by posterior reversible encephalopathy syndrome: a case report
Article information
Abstract
Posterior reversible encephalopathy syndrome (PRES) is a rare condition manifested by inflammation in certain areas of the brain. Rhabdomyolysis with acute kidney injury (AKI) complicated by PRES is rarely reported. A 26-year-old female presented with neurological symptoms, high blood pressure, and AKI. Her symptoms improved with blood pressure control, anticonvulsant drug medications, and renal replacement therapy. This case demonstrates that PRES should be considered in the differential diagnosis of patients who have rhabdomyolysis with AKI accompanied by neurological symptoms, including headaches and convulsions.
Posterior reversible encephalopathy syndrome (PRES) is an uncommon radiological condition characterized by headaches, altered mental status, seizures, visual problems, and typical magnetic resonance imaging (MRI) findings of posterior cerebral white-matter edema.1
Several medical conditions have been associated with PRES, including hypertension, immunosuppressive and cytotoxic drug administration, acute kidney injury (AKI), massive blood transfusion, mitochondrial disorders, trauma, eclampsia, thrombotic thrombocytopenic purpura, porphyria, polyarteritis nodosa vasculitis, sepsis, and systemic lupus erythematosus.2 Except for those who were taken immunosuppressive medication, the majority of patients displayed a reversible alteration along with blood pressure control.1,3 Patients prescribed such drugs show convulsions and visual disturbance when the blood pressure increases above the value at admission, but their symptoms improve after taking BP-lowering drugs.
Rhabdomyolysis is a sickness characterized by muscular necrosis and the release of intracellular muscle components into the bloodstream, and can have diverse causes such as a crush injury, medications, strenuous exercise, infection, and heat stroke.4 Damaged skeletal muscle breaks down rapidly in rhabdomyolysis, and some of the resulting products (e.g., the protein myoglobin) are harmful to the kidney and may lead to kidney failure. In rare cases dialysis may be needed to help the kidneys filter waste products while the patient recovers.
PRES may occur infrequently in rhabdomyolysis with AKI, and there are rare reports in the literature of rhabdomyolysis caused by influenza A, leptospirosis, and exercise.5-7 There are four previously reported cases of exercise-induced rhabdomyolysis with AKI complicated by PRES.7 PRES in rhabdomyolysis patients is rare in Korea. We report a rare case of PRES in a patient with AKI and exercise-induced rhabdomyolysis.
CASE
A 26-year-old female with no underlying illness visited the emergency room with nausea, general weakness for 4 days, and decreased urine volume for the past week. She had been on a diet and exercising for a few months, eating only one meal a day and walking every day for about 5 hours. At admission her BP was 136/86 mmHg, pulse rate was 98 bpm, body temperature was 36.8°C, and respiration rate was 20 breaths/min. She appeared ill, but did not have generalized edema.
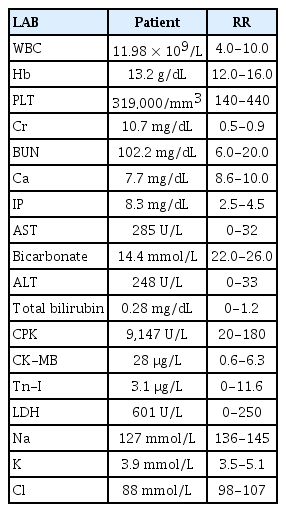
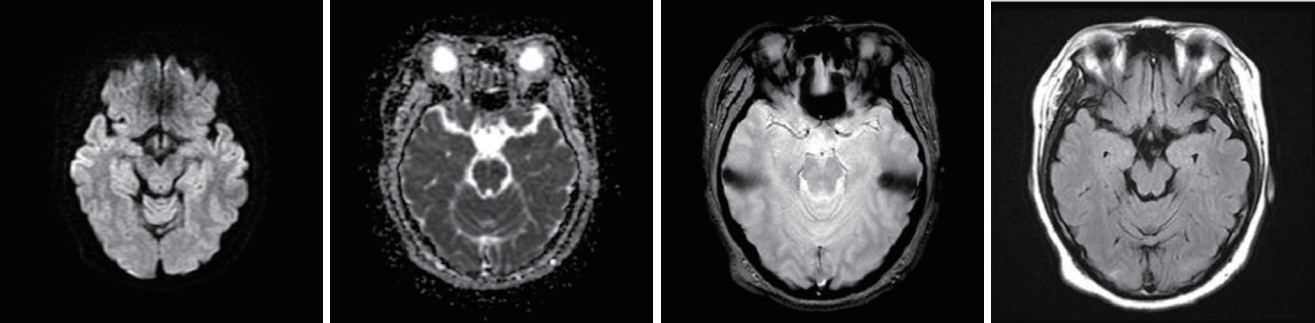
A physical examination revealed no abdominal or costovertebral angle tenderness, or other unusual findings. Her cardiovascular and respiratory systems were normal, and no neurological abnormalities were observed. The findings of peripheral blood tests performed at the time of admission are presented in Table 1. These blood tests revealed AKI due to rhabdomyolysis. Her oliguria, pulmonary edema, and uremic symptoms worsened, and she underwent emergency hemodialysis, after which her pulmonary edema, electrolyte disturbance, and metabolic acidosis improved. On the 6th day of hospitalization she experienced a severe headache, visual disturbance, high BP, and a seizure (Fig. 1). Brain computed tomography (CT) and diffusion MRI led to a diagnosis of PRES with subarachnoid hemorrhage (SAH) (Figs. 2, 3).
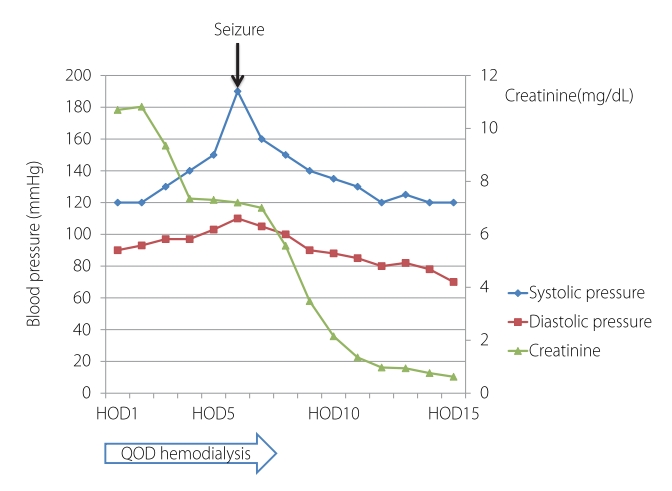
Blood pressure and creatinine level changes during hospitalization. HOD, hospital of day; QOD, every other day.
Initial diffusion-weighted magnetic resonance imaging of the brain showed increased fluid-attenuated inversion-recovery signals at the bilateral occipital lobes and bilateral posterior parietal lobes.
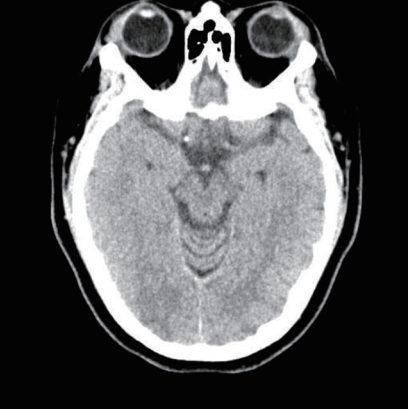
Initial brain computed tomography revealed subarachnoid hemorrhage at the right frontoparietal sulci.
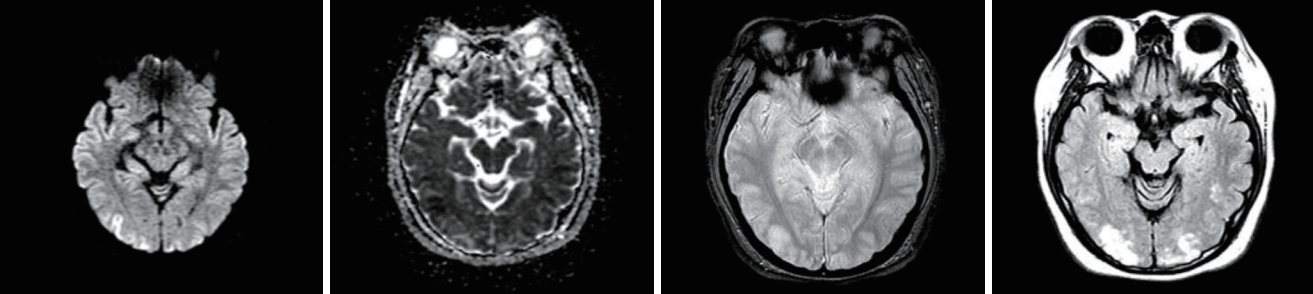
She was admitted to the intensive care unit and managed with BP control, anticonvulsant drug medications, and renal replacement therapy. After these treatments her neurological symptoms and AKI improved. The lesion had improved in the follow-up MRI performed 1 week later (Fig. 4). On the 15th day of admission her creatinine level normalized (0.76 mg/dL), and she was discharged. One month later her serum creatinine level was 0.68 mg/dL and there were no neurological symptoms.
DISCUSSION
PRES is a rare clinical syndrome characterized by vomiting, headaches, various visual abnormalities, confusion, and seizures. Neuroimaging reveals edema of the posterior regions of the brain, and all symptoms are reversible.1,8,9 Several conditions have been implicated as etiologies of PRES, including acute and chronic renal diseases, hypertensive encephalopathy, and vasculitides.10 The pathology underlying PRES has not yet been fully established. The conditions known to trigger PRES are heterogeneous, but vascular endothelial dysfunction is a key feature that is likely to represent a final common pathway leading to disordered cerebral autoregulation and the development of vasogenic edema.3 PRES has an anatomical preference for parieto-occipital regions of the brain because of less sympathetic innervation in the posterior cerebral circulation.7 White-matter regions (rather than cortical regions) are more vulnerable to hydrostatic edema due to their relatively loose structure.8 The present patient had rhabdomyolysis-induced AKI. On the 6th day of hospitalization, hypertension occurred concurrently with visual abnormalities and seizures, and we believe that this precipitated the PRES. Since the neurological symptoms and parieto-occipital edema improved after controlling BP, we concluded that the patient developed PRES-associated AKI and increased BP due to rhabdomyolysis.
Cerebrovascular CT alone has low specificity for diagnosing PRES. MRI, and in particular FLAIR (fluid-attenuated inversion-recovery) imaging, can be used to differentiate and diagnose mild cerebral edema around the cerebrospinal fluid by lowering the cerebrospinal fluid signal at low intensities compared with T2-weighted images.8 Because there is no specific treatment modality for PRES, treatments are focused on alleviating symptoms. The management of seizures and hypertension, together with the specific therapy for the underlying condition, is routine practice. No controlled trials have determined the best approach for treating these patients.7 Starting PRES treatment earlier will reduce the probability of long-term neurological effects. Treatment should center on careful fluid management and BP control with an intravenous antihypertensive such as nicardipine, labetalol, sodium nitroprusside, or hydralazine.7 Continuous invasive BP monitoring is advised since isolated measurements of BP may be misleading. Caution is required when using nitrates for BP control due to the possibility of exacerbating cerebral vasodilatation and edema.7 One distinguishing consequence of the present case relative to the previous cases is that the condition may be accompanied by cerebral hemorrhage that is not expressed by PRES alone.
In addition to brain MRI, brain workups such as brain CT and brain CT angiography will be needed. PRES with SAH was observed in the present case, and it was imperative to prevent the complications that caused the intracerebral hemorrhage. Therefore, nicardipine was used to control BP, with a target decrease of 20% during a change in consciousness. Also, since the PRES was related to breakdown of the blood-brain barrier, it was treated with dexamethasone intravenously and prophylactic anticonvulsant medication (intravenous valproate sodium at 600 mg twice daily).
This case report indicates that PRES must be included in the differential diagnosis for patients with exercise-induced rhabdomyolysis with AKI accompanied by neurological symptoms such as headaches and convulsions.
Notes
Conflicts of Interest
There was no conflict of interest.
Acknowledgements
Our research protocol received the Institutional Review Board (IRB) approval and was compliant with the Helsinki Declaration (IRB file number: 2019-08-026-004).