Abnormal spontaneous electromyographic activity in myasthenia gravis causing a diagnostic confusion: a case report and literature review
Article information
Abstract
Some cases of myasthenia gravis (MG) with abnormal spontaneous activity (ASA) in needle electromyography (EMG) have been reported, but the associated clinical characteristics remain to be fully elucidated. We report the case of a 36-year-old male with MG in whom ASA was observed. This study highlights that ASA may appear in needle EMG in patients with severe MG who predominantly have bulbar and/or respiratory involvement. Care is needed because this often accompanies myopathic features and can be misdiagnosed as myopathy.
Clinicians occasionally observe abnormal spontaneous activity (ASA) in needle electromyography (EMG) in patients with myasthenia gravis (MG), which is not a characteristic of MG. Although some cases of MG with ASA have been reported,1-9 its clinical characteristics remain to be fully elucidated. Here we report a case of MG with needle EMG findings of ASA.
CASE
A 36-year-old male presented to our hospital with a 1-week history of dysphagia and respiratory insufficiency. He had first noticed nasal speech and facial weakness 3 months previously, and ptosis and progressive limb weakness had occurred 1 month previously. The symptoms were not fluctuating or fatigable. At admission, the patient was in severe respiratory distress and had an oxygen saturation of 80%. Mechanical ventilation was initiated after tracheal intubation. A neurological examination revealed bilateral ptosis, facial weakness, dysphagia, nasal speech, neck muscle weakness (Medical Research Council [MRC] grades 0/5 and 1/5 in the neck flexors and extensors, respectively), and bilateral symmetric proximal dominant limb muscle weakness (MRC grade 3/5 proximally and 4/5 distally in the upper limbs, and 4-/5 proximally and 5/5 distally in the lower limbs). No muscle atrophy was detected anywhere in the body, including the tongue. Abnormal deep tendon reflexes, ophthalmoplegia, sensory deficits, and pathological reflexes were not observed. A nerve conduction study showed reduced compound muscle action potential amplitudes in the bilateral facial and ulnar nerves and in the right median nerve. Sensory nerve conduction was normal. Needle EMG revealed fibrillation potentials and positive sharp waves in the right genioglossus, flexor carpi radialis, peroneus longus, and cervical paraspinal muscles, and a few small-amplitude, short-duration motor unit potentials in the right genioglossus muscle (Fig. 1A). Repetitive nerve stimulation at 5 Hz elicited decremental responses in the orbicularis oculi (-39% at rest and -35.6% postexercise, Fig. 1B) and abductor digiti minimi (-33.9% at rest and -42.6% postexercise, Fig. 1C). The patient was positive for acetylcholine receptor antibodies (titer, 18.743 nmol/L; normal range, ≤ 0.5 nmol/L), had a normal serum creatine kinase level (124.1 U/L), and was negative for serum immunological markers, including anti-Jo-1 antibody. An anti-muscle-specific tyrosine kinase (MuSK) antibody test was not performed because it was not available in our center.
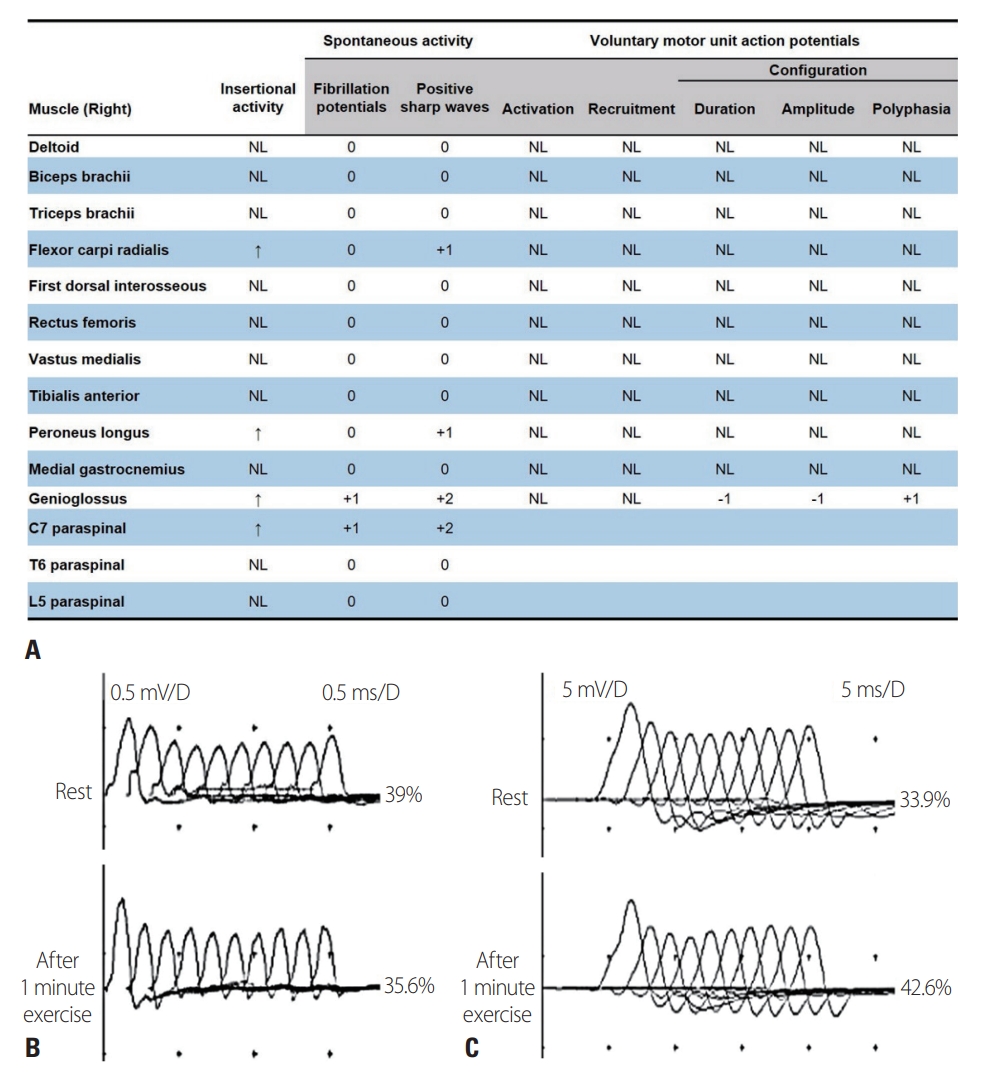
Electrophysiological findings for the patient. (A) Needle electromyography revealed fibrillation potentials and/or positive sharp waves in the right genioglossus, flexor carpi radialis, peroneus longus, and cervical paraspinal muscles, and myopathic motor unit potentials in the right genioglossus muscle. Repetitive nerve stimulation at 5 Hz elicited decremental responses in the orbicularis oculi (B, -39% at rest and -35.6% postexercise) and abductor digiti minimi (C, -33.9% at rest and -42.6% postexercise). NL, normal.
A myasthenic crisis was diagnosed. After 5 days of treatment with intravenous immunoglobulin, the patient’s neck, limb, and respiratory muscle weakness improved and he was successfully weaned from mechanical ventilation; however, mild bilateral ptosis persisted. Follow-up EMG performed 15 days after the initial assessment revealed no ASA or myopathic features. Chest computed tomography revealed an anterior mediastinal mass originating from the thymus. A transsternal thymectomy was performed, and a histopathological diagnosis of thymoma of World Health Organization type B3 was made. There was no further worsening of symptoms during the 35-month follow-up period, during which oral prednisolone and azathioprine were provided as maintenance therapy.
DISCUSSION
ASA was unexpectedly observed in needle EMG during a myasthenic crisis episode in a patient experiencing his first attack of MG. Previously reported cases of MG with ASA are summarized in Table 1.1-9 Previous reports of MG with ASA have described severe disease with predominantly bulbar and/or respiratory involvement. Most of the related studies showed myopathic motor unit potentials with ASA in needle EMG, which sometimes led to an initial misdiagnosis as myopathy.1,3,8,9 However, most patients had normal serum creatine kinase levels,1-4,6,7 and muscle biopsies did not reveal myopathic changes.1-3,6 Therefore, ASA and myopathic EMG findings in patients with MG reflect the blocking of muscle fibers due to severely impaired neuromuscular transmission rather than actual myopathy.1 This is supported by the findings that ASA and myopathic motor unit potentials were more prominent in the bulbar and neck muscles, which exhibited the most-severe weakness in our patient.
Needle EMG is usually not performed for diagnosing patients with fluctuating and fatigable weakness, which are typical of MG. However, as in our patient, needle EMG is often performed when fluctuating and fatigable weakness is absent in order to differentiate between MG and other diseases such as myopathy. In such patients, the unexpected observation of ASA and myopathic motor unit potentials in needle EMG can confuse the clinician and delay the diagnosis of MG.
Acetylcholine receptor antibody positivity was found in most of the previously reported cases,1-3,5-9 suggesting that these antibodies play a more important role than other antibodies in the development of ASA in MG. However, five (41.7%) of 12 patients with MuSK-antibody-positive MG in a previous study had ASA.10 Therefore, it is difficult to draw conclusions about the role of each antibody in the occurrence of ASA in patients with MG based on currently available data.
In conclusion, ASA may appear in needle EMG in patients with severe MG who predominantly have bulbar and/or respiratory involvement. Care is needed because this often accompanies myopathic features and can be misdiagnosed as myopathy.
Notes
Conflicts of Interest
The authors have no conflicts of interest to declare.

