Cerebral salt wasting syndrome caused by external lumbar drainage in a patient with chronic hydrocephalus
Article information
Abstract
In cases of hyponatremia induced by brain damage, it is important to distinguish between the syndrome of inappropriate anti-diuretic hormone secretion (SIADH) and cerebral salt wasting syndrome. A ventriculoperitoneal (VP) shunt is the standard treatment for hydrocephalus, and external lumbar drainage (ELD) is an option to evaluate the effect of a VP shunt. However, ELD has potential complications, such as subarachnoid hemorrhage, meningitis, and rarely hyponatremia. Therefore, we report a case of a patient with cerebral salt-wasting syndrome resulting from ELD to treat normal-pressure hydrocephalus during the rehabilitation of acute ischemic stroke.
Hyponatremia is a frequent electrolyte abnormality in patients with brain damage or diseases such as stroke, traumatic brain injury, and brain tumors. Hyponatremia may be caused by a syndrome of inappropriate anti-diuretic hormone secretion (SIADH) or cerebral salt wasting syndrome (CSWS).1-3 SIADH and CSWS present similar clinical manifestations; however, their pathophysiology and treatments are completely different. Inappropriate treatment can aggravate symptoms and cause mental changes, seizures, and even death. A rare cause of hyponatremia is the external lumbar drainage (ELD) of cerebrospinal fluid (CSF), which is used to predict the effectiveness of ventriculoperitoneal (VP) shunts in patients with symptomatic hydrocephalus.2 Here, we report a case of a patient with CSWS resulting from ELD to treat normal-pressure hydrocephalus during rehabilitation for ischemic stroke.
CASE
An 86-year-old woman visited the emergency center for right-sided motor weakness and was diagnosed with cerebral infarction of the left pons and right parietal lobe. She was hospitalized for the management of the acute phase of stroke with dual antiplatelet therapy. When transferred to the rehabilitation department, she showed mild cognitive deficits with a Mini-Mental State Examination (MMSE) score of 21/30. The patient’s language function was normal. Motor function of the right upper and lower extremities was Medical Research Council (MRC) grade 2/5. The left-side motor and sensory functions on both sides were intact. Despite motor weakness on the right side, the patient could stand with support for right knee extension.
History taking revealed that she had been diagnosed with normal-pressure hydrocephalus 5 years ago (2014) (Fig. 1). Her symptoms were progressive gait disturbance and urinary incontinence. Brain magnetic resonance imaging performed in 2014 showed hydrocephalus, with an Evans ratio of 36. The Seoul Neuropsychological Screening Battery showed cognitive deficits in multiple domains, such as set-shifting, visuospatial function, executive function, and visual memory. Alzheimer’s dementia was ruled out because there was no progression of the cognitive deficits. Apo E genotyping (E3/E3 [72%] and E4/E4 [0.7%]) and amyloid positron emission tomography results also suggested that it was not an Alzheimer’s dementia. Based on these findings, she was diagnosed with normal-pressure hydrocephalus.
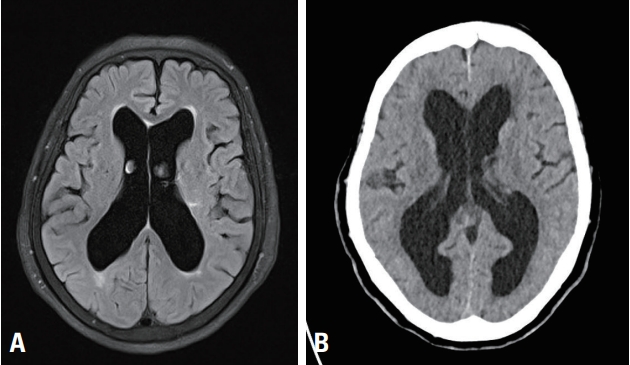
(A) Image showing hydrocephalus with Evans ratio 36 at 2014 on flair brain magnetic resonance imaging. (B) Image showing hydrocephalus with Evans ratio 40 at 2019 on brain computed tomography.
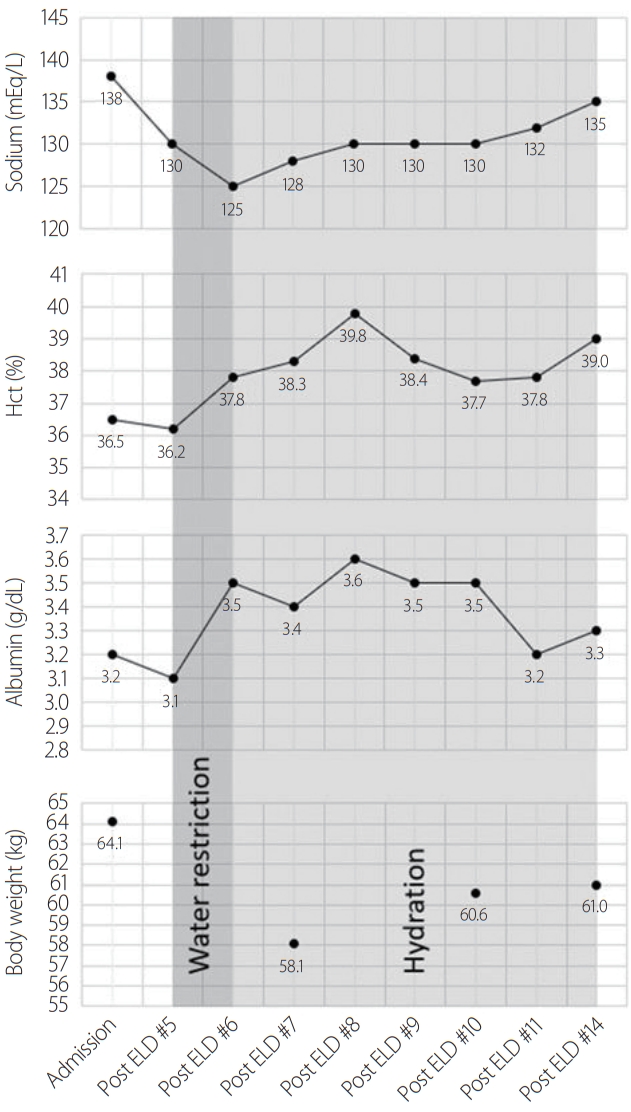
After 7 days of rehabilitation, her compliance worsened due to poor motivation, and she could not stand without support despite her increased motor function from MRC 2/5 to 3/5. In addition, brain computed tomography (CT) showed progression of hydrocephalus in comparison with the previous brain CT (2014) as Evan’s ratio increased from 36 to 40 (Fig. 1). As we considered that her symptom aggravation was due to hydrocephalus, we planned to perform a VP shunt to treat the hydrocephalus. To assess the potential effect of the VP shunt, an ELD for 3 days with 50 mL daily drainage. On the first day of ELD, the opening pressure was normal (12 cmH2O), and 50 mL of CSF was drained. On the second day, 38 mL of CSF was drained. On the third day, EDL failed because of severe pain during the procedure.
Three days after the ELD, her general condition worsened. Food intake was reduced, and the response to stimuli decreased. The MMSE score could not be checked because of the presence of abulia. Neurological examination, including motor, sensory, and neurological reflexes, did not show any specific changes. Laboratory data showed the development of hyponatremia (135–130 mEq/L) and low blood osmolarity (262 mOsm/kg). However, the urine osmolarity was normal (508 mOsm/kg). The adrenocorticotropic hormone stimulation and pituitary function tests, which can diagnose hyponatremia by endocrine dysfunction, were normal. Serum antidiuretic hormone (ADH) level was in the normal upper range (6.6 pg/mL), although the serum osmolarity was low (262 mOsm/kg). The patient was taking aspirin, clopidogrel, statin, bethanechol, and tamsulosin for acute ischemic stroke and neurogenic bladder; however, the cause of hyponatremia was unknown. Blood pressure and pulse rate were normal (136/87 mmHg, 71 beats/min), and there were no signs of hypovolemia, such as a dehydrated tongue or axilla. The patient was diagnosed with SIADH, and water restriction treatment was initiated.
Despite water restriction treatment, her serum sodium level dropped to 125 mEq/L, and her body weight decreased from 64 to 58 kg. Echocardiography performed 7 days after ELD showed hyperdynamic left ventricular systolic function due to a decreased effective arterial blood volume. Laboratory findings showed increased albumin, potassium, and hematocrit levels. These clinical and laboratory findings suggested that she had hypovolemia caused by CSWS rather than SIADH. We stopped water restriction and started intravenous hydration.
After hydration with 1 L of normal saline for 5 days, her serum sodium level was restored from 130 to 136 mEq/L. Her general condition improved, with an increase in body weight of 4 kg in 12 days. Her mental status became alert, and her response to the stimuli was restored. The MMSE score improved to 22/30 (Fig. 2).
DISCUSSION
Hyponatremia can be caused by damage to the central nervous system (CNS), such as stroke, traumatic brain injury, or CNS surgery.4 For clinical diagnosis, it is critical to distinguish between CSWS and SIADH.
Related to CNS damage, SIADH is known to occur with increased ADH secretion. Brain damage increases intracranial pressure (ICP), which reduces cerebral perfusion pressure (CPP). ADH secretion increases to compensate for the decreased CPP by elevating the mean arterial pressure. Furthermore, hypothalamic injury can result in excessive ADH secretions.5-7 In contrast, CSWS is caused by malfunction of the CNS, leading to an incorrect kidney neural input signal, creating hypovolemia and hyponatremia, which arises from excessive secretion of brain natriuretic peptide, atrial natriuretic peptide (ANP), and suppression of renin secretion.4,8
Patients with SIADH and CSWS have similar clinical manifestations and laboratory findings. However, the treatments for these two diseases are completely different. Inappropriate treatment can aggravate hyponatremia. Therefore, an initial differential diagnosis is important for proper treatment.
An important point in the differential diagnosis is the extracellular fluid volume. In the CSWS, the extracellular fluid volume decreased. In contrast, extracellular fluid volume was increased in SIADH. In CSWS, brain damage causes abnormalities in the neural input to the kidney, leading to a negative salt balance. Consequently, the effective arterial volume decreased, resulting in weight loss. Serum albumin and hematocrit levels increased because of the decreased effective arterial volume. In addition, serum sodium and osmolarity decreased due to a negative salt balance.
In cases of SIADH, inappropriate secretion of ADH can lead to increased serum ADH levels. However, in CSWS, serum ADH levels can be masked as normal or increased. A low sodium level, leading to effective arterial volume loss, is compensated for by an increase in ADH secretion. Therefore, it is essential not to differentiate between SIADH and CSWS based solely on serum ADH levels.4
In our case, hyponatremia started after CSF tapping. Rapid weight loss and echocardiographic findings confirmed a decreased effective atrial blood volume. Worsening hyponatremia during fluid restriction at the early stage of hyponatremia and improvement of sodium levels after massive hydration and salt replacement therapy were both compatible with CSWS.
In clinical practice, ELD is considered to predict the effect of VP shunting in patients with normal-pressure hydrocephalus, who show symptoms of urinary incontinence, gait disturbance, and cognitive impairment. The pathophysiology of hyponatremia caused by ELD is poorly understood. It is thought to be related to sodium loss caused by CSF leakage,9,10 and the amount of CSF loss is related to the severity of hyponatremia.11,12 Another study supports the hypothesis that CSF loss breaks the homeostasis of CSF osmolarity and results in a reset osmostat with SIADH.13 There are several case reports of CSWS following neuro-interventions, such as ELD, VP shunt, and brain surgery.3,14-16 However, its pathophysiology has not been clearly understood in previous studies. There is a hypothesis that ICP may increase if there is a problem in CSF circulation due to neuro-intervention, which may promote the secretion of ANP to decrease ICP17,18 and could cause CSWS.4,8
In our case, the patient showed symptoms such as poor motivation and general weakness, and the brain CT findings confirmed the aggravation of hydrocephalus. Therefore, intervention for hydrocephalus was performed because the aggravation of hydrocephalus could explain the patient’s symptoms. However, there is a possibility that hydrocephalus was not the reason for the patient’s symptoms since hydrocephalus usually does not cause acute symptoms, as in our patient.
Symptoms that occurred after the progression of hyponatremia were severe and showed different characteristics. While the patient initially presented with general weakness and lack of motivation, the major symptoms were abulia, lethargy, and irritability. The pre-and post-hyponatremia symptoms could be due to different etiologies.
In our case, the serum sodium level was within the normal range upon admission, and hyponatremia occurred 5 days after ELD. These findings suggest that the cause of CSWS is ELD. However, it is thought that the maintenance of sodium homeostasis is further inhibited by performing ELD in a state where sodium metabolism and equilibrium are abnormal due to chronic hydrocephalus and acute stroke.
ELD to evaluate the effect of a VP shunt could aggravate hyponatremia and the patient’s general condition. Therefore, we must be careful with VP shunt and ELD in patients with CNS damage and take proper measures when secondary hyponatremia is present after CNS damage by taking SIADH and CSWS into account.
Notes
Conflicts of Interest
The authors have no conflicts to disclose.