Spectrum of nitrous oxide intoxication related neurological disorders in Korea: a case series and literature review
Article information
Abstract
Background
Nitrous oxide (N2O) is used in surgery and dentistry for its anesthetic and analgesic effects. However, neurological and psychiatric manifestations of N2O abuse have been increasingly reported among Korean adults. The aim of this study was to demonstrate laboratory findings of N2O abuse in Korean patients.
Methods
Patients diagnosed with N2O-induced neuropathy or myelopathy from August 2018 to December 2019 were enrolled. Their clinical presentations and laboratory and imaging findings were analyzed.
Results
Sensory changes and limb weakness were present in nine of the enrolled patients. The laboratory findings revealed that seven patients had high homocysteine levels and five had high methylmalonic acid levels in their blood. Nerve conductions studies indicated that axonal neuropathy was present in four cases and longer F-wave and Hoffman’s-reflex latencies were present in two cases. Signal changes in cervical spine imaging occurred in five patients, while two had normal results.
Conclusions
Chronic N2O abuse can cause neurological damage or psychiatric problems. Because N2O is illegal for recreational use in Korea, patients tend to hide their history of use. Even though the spinal imaging results were normal, clinicians should consider the possibility of N2O use, and further electrophysiological tests should be applied for precise evaluations.
INTRODUCTION
Nitrous oxide (N2O) is a colorless and nonflammable gas that is used in surgery and dentistry due to its anesthetic effects. N2O has recently been misused for recreational purposes due to its euphoric effects when inhaled. The recreational use of N2O is currently prohibited in Korea due to its negative clinical effects, such as inducing vitamin B12 deficiency1 or mechanisms that lead to thrombosis.2 However, N2O is still available in whipped-cream canisters or small bulbs that are predominantly used for recreation by young people.3 Reported cases of neurological and psychiatric manifestations of N2O abuse have recently increased among adults in Korea.4
The neurological complications associated with N2O abuse usually manifest as subacute combined degeneration (SCD) of the spinal cord. N2O deactivates the enzyme methionine synthase by inactivating methylcobalamin and impairing the methylation of myelin sheath proteins, leading to spinal cord degeneration and myelopathy.4 These processes can also cause peripheral nervous polyneuropathies. Several clinicians in Korea have reported various clinical symptoms and abnormal findings in tests after N2O inhalation, such as in electrophysiological examinations and magnetic resonance imaging (MRI).5 Additionally, vitamin B12 deficiency tends to the induction of hyperhomocysteinemia, which in turn induces thrombosis.2 There are numerous previously reported cases of hyperhomocysteinemia inducing deep vein thrombosis or pulmonary thromboembolism (PTE).2,6
The use of illegal drugs such as cocaine, heroin, methamphetamine, and cannabis is reportedly lower in Korean than in other countries.7 Illegal drug usage is therefore considered by Korean clinicians less when they are determining symptom etiology. Since it is rare for patients to report their N2O use, diagnosing patients with atypical clinical manifestations is also difficult. A good understanding of the clinical manifestations of N2O usage is therefore important. Here we report nine patients with neurological manifestations and abnormal laboratory findings following N2O abuse.
MATERIALS AND METHODS
Patient sample and clinical measurements
We retrospectively analyzed clinical data from nine patients diagnosed with N2O-induced neuropathy or myelopathy. This hospital-based case-series study investigated patients who presented at the Hanyang University Hospital (a tertiary referral medical center) in Seoul, Korea from August 2018 to December 2019. All patients underwent routine laboratory tests including a complete blood count, coagulation test, electrolyte test, and routine urine analysis. Considering the effects of N2O on vitamin B12 metabolism, serum methylmalonic acid (MMA), homocysteine, and vitamin B12 levels were also examined. Electrophysiological tests such as a nerve conduction study (NCS) or electromyography were applied to eight patients. Spinal cord MRI, computed tomography (CT) scan with contrast enhancement, and chest CT angiography had been performed on seven, one, and one patient, respectively. Neurological examinations were performed, with the motor power of all four limbs measured according to the Medical Research Council grading system. Ethical approval was obtained for this study from the Institutional Review Board of the Hanyang University Hospital (IRB No. 2020-03-008).
Electrophysiological examinations
Standard motor and antidromic sensory NCSs were performed bilaterally on four motor nerves (median, ulnar, posterior tibial, and fibular) and three sensory nerves (median, ulnar, and sural) of eight patients. This analysis evaluated the terminal latency, compound muscle action potential amplitude (CMAP), and conduction velocity of each nerve. We defined abnormal results as changes of at least 20% from the lower conduction velocity and CMAP limits, and from the upper terminal latency limit. Demyelinating NCS patterns were defined according to the guidelines from the American Association of Neuromuscular and Electrodiagnostic Medicine.8 F-wave latency was measured after supramaximal motor nerve stimulation, which identified ten F-waves. Hoffmann’s reflex (H-reflex) was recorded from the soleus when stimulating the tibial nerve.
Literature review
Literature searches were performed of the MEDLINE and PubMed databases using the search terms “neuropathy N2O” and/or “myelopathy N2O” and including case reports published between 2006 and 2019 in South Korea. The reference lists of these reports were considered secondary sources. Cases without postmortem analyses or NCS results were excluded.
RESULTS
Nine patients aged between 23 and 35 years visited our hospital from August 2017 to December 2019 (Table 1). All patients had a history of N2O inhalation during the 6 months prior to admission. The interval between the last N2O inhalation and symptom onset varied from 2 weeks to 4 months, while the interval between symptom onset and hospital visit varied from 3 days to 1 month, with the majority of patients visiting 1 week after symptom onset. Five patients were female. Sensory change was the initial symptom of all patients. These sensory symptoms varied between patients and included an ascending tingling sensation in both legs (patient no. 1), numbness in both legs (patient no. 3 and 7), and acroparesthesia (patient no. 2, 4, 5, 8, and 9). Five patients experienced muscle weakness, three of which only had lower limb weakness (patient no. 3, 6, and 9), and two had both upper and lower limb weakness (patient no. 1 and 4). Muscle weakness only in the ankles was found in patient no. 5. One patient had dyspepsia. None of the patients were vegetarians or had previously received gastrointestinal surgery.
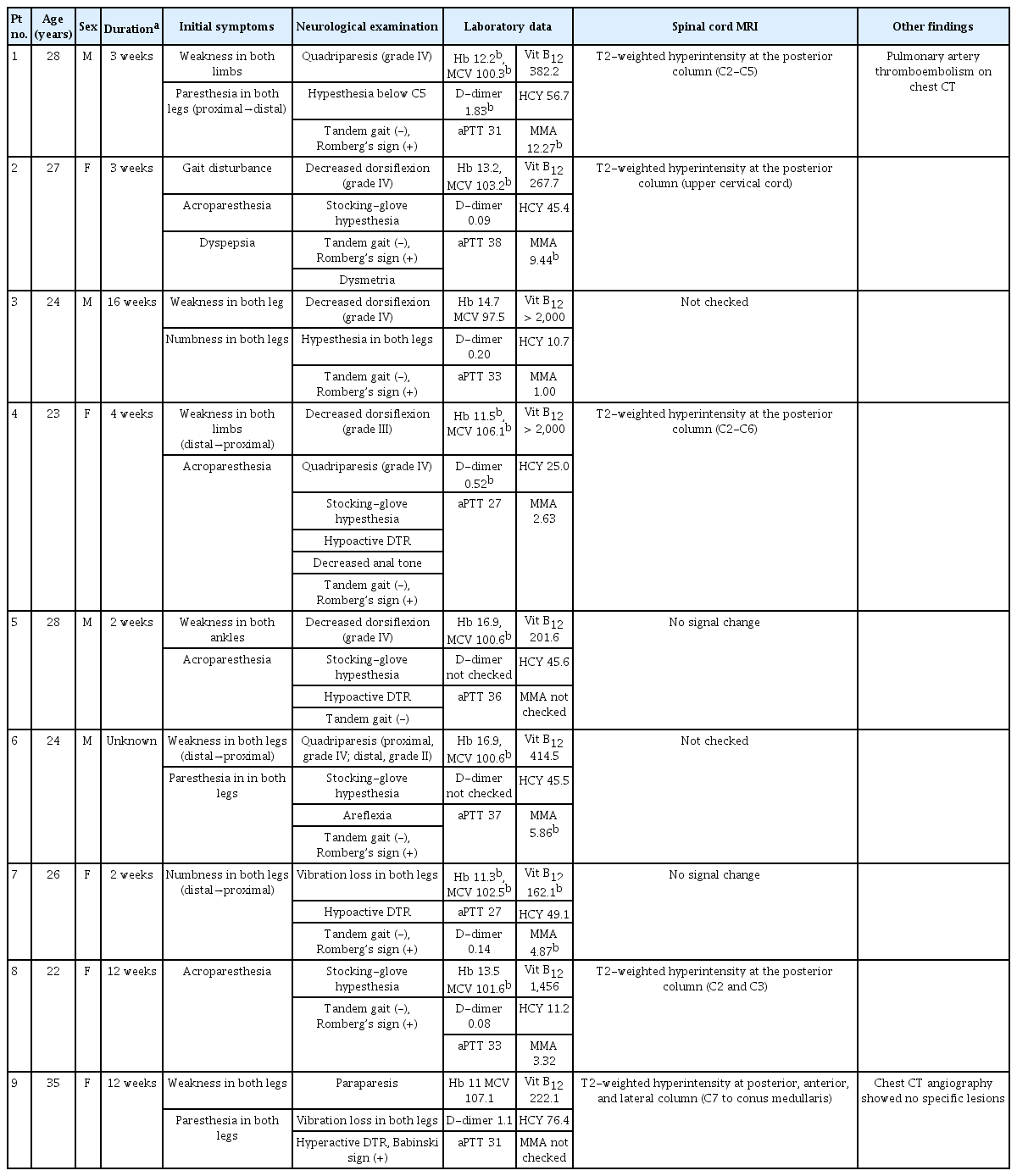
Clinical features and laboratory findings of nine patients with neurological effects from (N2O) intoxication
Neurological examinations indicated that seven of the nine patients had limb paresis. Three had mild weakness in ankle dorsiflexion and the other four had quadriparesis. Sensory changes were detected in patient no. 1, while patient no. 7 and 9 had a loss of vibration sensation in both legs. Among the other patients, six had stocking-glove hypesthesia (patient no. 2, 4, 5, 6, 8, and 9). Hypoactivity of the deep tendon reflex (DTR) occurred in four cases (patient no. 4, 5, 6, and 7), four had normoactive DTR, and one had hyperactive DTR (patient no. 9). Patient no. 2 had dysmetria of the upper limbs and all patients except no. 5 showed abnormal results in the Romberg test. All patients had an impaired tandem gait. Digital rectal examinations indicated that patient no. 4 had decreased anal tone.
Laboratory findings revealed low hemoglobin levels and large mean corpuscular volumes in four and six patients, respectively. Only one patient had a low serum vitamin B12 level (patient no. 7). Elevated serum homocysteine levels were indicated in seven cases (patient no. 1, 2, 3, 5, 6, 7, and 9), and five patients had high blood MMA levels.
NCSs were performed on eight patients (Table 2). Among them, four had axonal neuropathies (patient nos. 3, 4, 5, and 6) and one had longer F-wave and H-reflex latencies in both lower limbs (patient no. 7). Seven patients had a decreased sural nerve conduction velocity or longer H-reflex latency. Most patients had more abnormalities in the lower limbs than in the upper limbs, distal limb regions were affected more than were proximal regions, and sensory nerves were involved more frequently than were motor nerves. Demyelination was not observed in any patient. The reference values and raw data of patients' nerve conduction study are shown respectively on Supplementary Table 1, 2.
Seven patients received spinal MRI, five of whom had signal changes in the dorsal column of the cervical spine, as indicated in T2-weighted images (Fig. 1). Patient no. 9 had concurrent hyperintensity lesions in the anterior, lateral, and posterior spinal column (Fig. 2). Two patients (patient no. 5 and 7) showed normal results in spinal MRI.
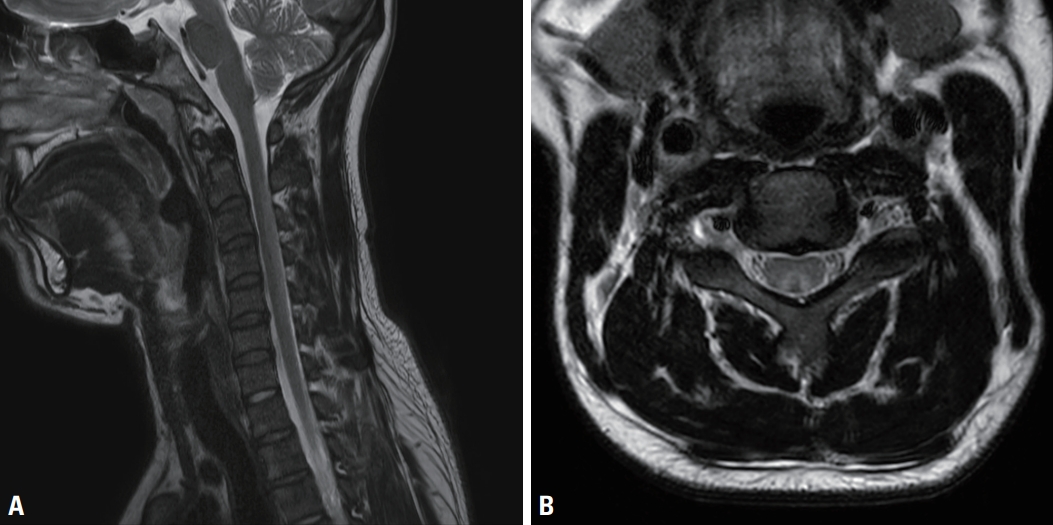
T2-weighted cervical spinal magnetic resonance imaging (patient no. 1). (A) In the sagittal series, abnormal hyperintensities were observed in the dorsal cervical spinal cord (C2-C5) without enhancement. (B) In the axial series, high signal intensity (inverted-V sign) and cord swelling were observed.

T2-weighted spinal magnetic resonance imaging (patient no. 9). (A) T2-weighted sagittal image, indicating high signal intensities within the posterior spinal cord from C7 to the conus medullaris. (B) T2-weighted axial image indicating high signal intensities in the anterior, lateral, and posterior spinal column.
All patients received hydroxocobalamin as a vitamin B12 supplement. Three patients received additional steroid therapy. Intravenous immunoglobulin G was administered to one patient (patient no. 5) due to their observed clinical features being similar to those associated with early Guillain-Barré syndrome. Patient no.1 suffered from N2O addiction and depression, so they received psychiatric consulting.
A literature search identified five articles describing SCD attributed to recreational N2O abuse, including three male and seven female cases. All of these cases had gait disturbances or sensory changes as initial symptoms accompanied by signal changes in cervical MRI. NCSs indicated neuropathic patterns or early-stage polyneuropathy. One patient presented with incidental PTE and lung infarction. Table 3 summarizes the details of the published cases.5,9,10
DISCUSSION
N2O is known to selectively oxidize folate and vitamin B12, rendering them inactive and unable to degrade homocysteine into methionine, which is required for normal myelin production.11 Active vitamin B12 is also required to convert MMA into succinyl-CoA. Serum levels of both homocysteine and MMA are elevated during vitamin B12 deficiency. Previous in vitro studies have demonstrated the cytotoxic effect of homocysteine on cortical astrocytes and that of MMA on primary neuronal cultures.12,13 This interference with vitamin B12 metabolism could lead to demyelination in the central or peripheral nervous system and also to megaloblastic anemia. These pathophysiological mechanisms result in N2O intoxication being associated with clinical features such as numbness in the extremities, ataxia, and psychomotor symptoms (including impaired memory function and depression).14
There were marked variations in clinical symptoms, laboratory findings, electrophysiological findings, and MRI results associated with N2O usage among the cases examined in this study. Sensory nerve fibers are generally affected more frequently by toxins.15 Similar to other toxic neuropathies, we found that all of our patients experienced sensory changes in their distal limbs, and that these changes were correlated with NCS abnormalities. Motor weakness was observed in 50% of cases, but motor NCS abnormalities were not found to be correlated with these symptoms. In two cases (patient no. 1 and 7), only F-wave and H-reflex latency changes were identified. These findings resembled the early clinical features of Guillain-Barré syndrome, but our results indicated no demyelinating patterns. Considering the relationships between N2O, vitamin B12 deficiency, and hypomyelination, these axonal patterns detected through NCSs suggest that an alternative mechanism of N2O directly contributes to axonal damage.16
Most of the patients had high serum homocysteine or MMA levels despite normal vitamin B12 levels. Due to the illegality of recreational N2O use in Korea, most patients with neurological symptoms were assumed to have ingested vitamin B12 prior to visiting our hospital. A previous case report indicated that homocysteine and MMA levels could be normalized by vitamin B12 treatment.17
MRI findings in previously reported cases of N2O intoxication related to SCD have frequently identified symmetric bilateral T2-weighted hyperintensity lesions at the posterior spinal cord. This specific finding is known as the “inverted-V sign.” In the present case series, the MRI findings of about half of the patients were compatible with SCD. However, two patients who had no detected MRI signal changes had abnormal NCS results, which represents the first report of this phenomenon in Korea. One patient also had concurrent involvement in the anterior, lateral, and posterior spinal column.
There have been several reports of the deleterious effects of N2O abuse in Korea. Kwoun et al.5 reported the first case of myeloneuropathy following chronic N2O abuse in 2003. In that report, the patient had paresthesia in both distal limbs and ataxia. Spinal MRI indicated high signal intensities from segments C2 to C5 on a T2-weighted image. Furthermore, NCSs indicated a demyelinating neuropathic pattern. Serum vitamin B12 levels were low and those of homocysteine were high. Two other cases of SCD caused by N2O gas were reported by Kwon et al.18 in 2019. Both patients had sensory changes in their limbs and the inverted-V sign was visible in T2-weighted spinal MRI. NCSs indicated axonal motor polyneuropathy in that case. In all previous cases of N2O intoxication in Korea, spinal cord MRI indicated signal changes similar to those observed in SCD. Unlike previously reported cases, we found two patients who had abnormal NCS results despite having normal spinal MRI results. We therefore suggest that clinicians need to consider the possibility of N2O inhalation in patients whose NCSs produce axonal degeneration findings, even if signal changes are not observed in spinal cord MRI.
The present study has revealed that N2O abuse may be associated with a wide range of clinical features, including psychiatric problems such as addiction and depression, especially among adolescents and young adults. Due to the illegality of recreational N2O use in Korea, patients tend to hide their history of N2O use or inaccurately report symptom onset. Addressing this issue requires treatments for neurological deficits, the careful recording of the medical histories, and psychiatric support for patients.
Our study had several limitations. First, its retrospective design made it difficult to precisely evaluate and quantify the data analyzed for each patient. Second, this study included only nine cases from a single center, so it might not be representative of all patients experiencing N2O intoxication in Korea. Third, all patients had supplemented their vitamin B12 intake before the clinical evaluations, which makes it difficult to accurately interpret the results. Fourth, the patients tended to not reveal their history of N2O inhalation because it is currently illegal to use N2O gas for recreational purposes in Korea. It was therefore difficult to evaluate the correlations between clinical features and the exact amount and duration of N2O inhalation.
The clinical manifestations of N2O intoxication related to neurological disorders varied between the included patients, but had a general commonality. Several clinical indicators may include young adults with progressive subacute weakness and lower-extremity-dominant sensory changes. Increased serum MMA, homocysteine, axonal-type sensorimotor polyneuropathy patterns in electrophysiological tests, and predominant posterior column involvement observed in MRI may indicate the presence of N2O intoxication related to neurological disorders. Careful evaluations of the clinical history of N2O abuse are therefore warranted when these clinical findings are observed.
Notes
Conflicts of Interest
The authors have no conflicts to disclose.
Acknowledgements
This work was supported by the research fund of Hanyang University(HY-202100000001136).


