Electrognostic findings of Guillain-Barré syndrome
Article information
Abstract
The electrodiagnostic findings in Guillain-Barré syndrome (GBS) play important roles in both understanding its pathophysiology and its diagnosis. Only demyelinating neuropathies were thought to be present when GBS patients were first diagnosed in Western countries, but the concept changed when many axonal GBS patients were reported in Asia. Reversible conduction failure was subsequently revealed, and it was recognized as a pathophysiologic continuum of axonal GBS. Thus, the electrodiagnostic findings in GBS have had a profound effect on the history of this disease.
INTRODUCTION
Guillain-Barré syndrome (GBS) is normally the first condition considered when patients experience acute-onset progressive motor weakness after an upper respiratory or gastrointestinal infection.1 GBS is generally easy to diagnose when it occurs with the typical clinical presentation such as ascending paralysis and hyporeflexia. However, this is not always the case, which can make the accurate diagnosis of GBS challenging for neurologists who experience patients in the early stage of the disease. In such cases the presence of albuminocytologic dissociation in cerebrospinal fluid and abnormal findings in nerve conduction studies (NCS) can be clues.1 The most-representative NCS findings in GBS include motor and/or sensory nerve conduction slowing or block, prolonged distal motor latency (DML), and prolonged or absent F-wave, but the findings are usually normal in the early stage (up to 13%).2-4 Therefore, serial NCS tests of the upper and lower limbs can be helpful for ensuring that diagnoses are accurate.3
The history of GBS has been greatly influenced by the understanding of electrophysiological changes.5 GBS was first diagnosed in Western countries and presented as a demyelinating neuropathy in most patients, which resulted in it being accepted as a concept same with acute inflammatory demyelinating polyneuropathy (AIDP).3,6 However, axonal GBS is now known to cover a spectrum of conditions, including reversible conduction failure (RCF). This means that electrophysiological findings can be used to understand the pathophysiology of GBS.7,8
EARLY ABNORMAL ELECTRODIAGNOSTIC FINDINGS IN GBS
In 1985, Albers and colleagues reported notable motor and sensory NCS and F-wave abnormalities occurring over time at least 3 weeks after symptom onset in GBS patients.4 Their report was also the first on the sural sparing phenomenon, with a normal sural sensory nerve action potential (SNAP) but an abnormal median SNAP, which has been accepted as a highly characteristic finding of GBS.
However, it is still important to understand the abnormal NCS findings in the early stage of GBS. The NCS findings are generally normal during the first 4 days, but after 1 week almost all patients begin to exhibit abnormal findings, and 50-65% of patients can be diagnosed as demyelinating polyneuropathy based on electrodiagnostic criteria.9-11 H-reflex abnormality occurs first (in 98-100% of patients), although there are differences depending on the studies, with abnormal F-waves, sural sparing, prolonged DML of motor nerves, and reduction of conduction velocities (CVs) occurring relatively early.10-12
Typically 10-20% of early-stage GBS patients exhibit normal peripheral NCS findings but abnormal F-wave and H-reflex findings.13,14 This is a good indicator to explain the slowing of the proximal portion of the peripheral nerves due to destruction or malfunction of the blood-brain barrier around their roots, which is considered to be the initial process in GBS.15,16
In 2000, Kuwabara and colleagues reported on the findings of follow-up NCS and anti-ganglioside-antibody tests–which is a well-known pathogen of axonal GBS–in 12 patients with isolated abnormal F-waves in the initial NCS.17 In follow-up NCS, six patients had returned to normal, four had progressed to acute motor axonal neuropathy (AMAN), and two were lost to follow-up. Anti-ganglioside antibodies were present in 11 of these patients (92%). Those authors suggested that an isolated abnormal F-wave is indicative of an antibody-mediated proximal motor conduction block (CB).
HISTORY OF THE ELECTRODIAGNOSTIC CRITERIA OF GBS
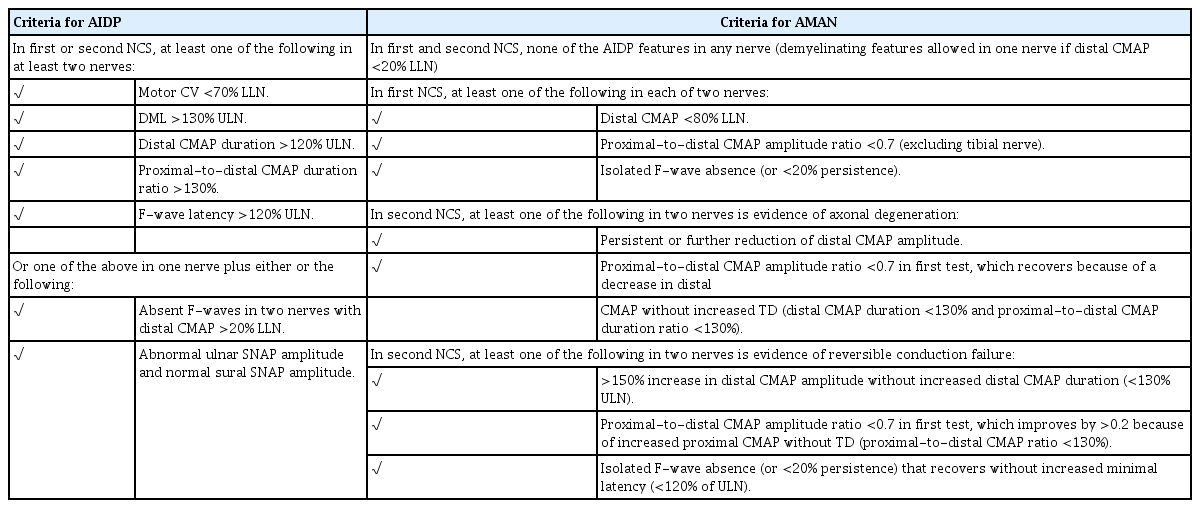
The electrophysiological characteristics of GBS were summarized for the first time in 1978, and clear cutoff values were first proposed in 1985.4,15 According to the Albers criteria, AIDP can be diagnosed if at least one of the following features is present in two or more nerves: 1) CV of less than 95% of the lower limit of normal if the amplitude exceeds 50% of the lower limit of normal, or of less than 85% of the lower limit of normal if the amplitude is less than 50% of the lower limit of normal. 2) DML exceeding 110% of the upper limit of normal if the amplitude normal, or exceeding 120% of the upper limit of normal if the amplitude is less than the lower limit of normal. 3) Evidence of unequivocal temporal dispersion (TD) or a proximal-to-distal amplitude ratio less than 0.7. 4) F-response latency exceeding 120% of the upper limit of normal.
At the time there was no definition of the compound muscle action potential (CMAP) duration, and so CB was defined based on the CMAP amplitude ratio, while the definition of TD was not clear.4,18 Cornblath and colleagues subsequently proposed new research criteria that modified the diagnostic criteria of chronic inflammatory demyelinating polyneuropathy. At least three parameters must be present in two or more nerves for a diagnosis, and the cutoff values are more strict than Albers criteria, which meant that these criteria showed the lowest sensitivity among the GBS diagnostic criteria (21-39%).12,19,20
Dozens of acute-onset patients with flaccid paralysis appear every summer in north China. At first glance they have symptoms and signs similar to those of AIDP, but these patients show completely different electrophysiological findings from AIDP, with only reduced CMAP amplitudes and without prolonged DML or decreased CV.2,6,21 Therefore, Ho criteria as well as Hadden criteria were proposed to diagnose these axonal GBS cases, both of which were modified from the Albers criteria. The Ho criteria introduced TD instead of CB, and the Hadden criteria introduced the concept of CB instead of TD (Table 1). Both criteria are still the most widely used for the electrophysiological classification of GBS.2,4,6
RCF AND ACUTE MOTOR CB NEUROPATHY
Capasso et al.22 reported two very interesting patients who exhibited flaccid paralysis after Campylobacter jejuni infection. The initial NCS revealed motor CB with normal or mild reduced distal CMAP amplitudes, but the CB disappeared within 2-5 weeks due to the proximal CMAP amplitudes recovering rapidly. However, TD and polyphasic patterns, which are characteristics of demyelinating neuropathy, were not seen, and the clinical symptoms recovered rapidly and the patients showed a good prognosis. This condition was called acute motor CB neuropathy (AMCBN) and described as ‘arrested AMAN’ or ‘partial AMAN.’ This transient CB (now called RCF) occurred mainly in patients carrying anti-ganglioside antibodies (Fig. 1).23
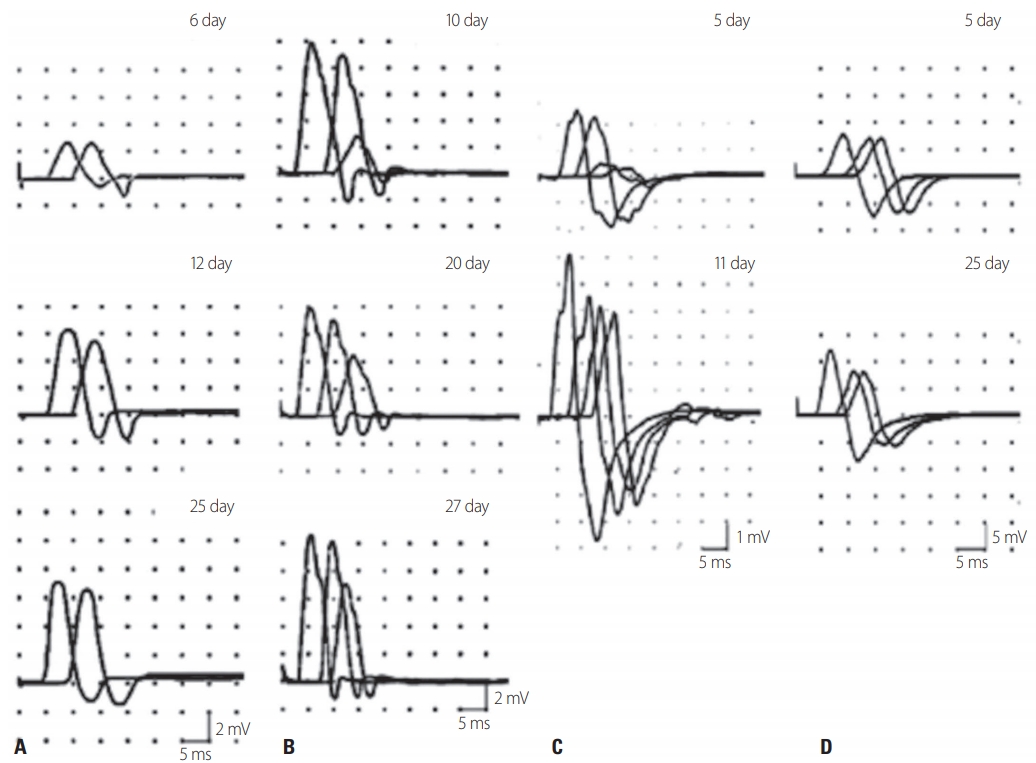
Types of reversible conduction failure (RCF). (A) Distal RCF in the median nerve. On day 6, distal and proximal compound muscle action potential (CMAP) amplitudes were reduced without prolonged distal motor latencies. On day 25, the distal CMAP amplitude was greatly increased without the development of temporal dispersion (TD). (B) RCF in an intermediate nerve segment of the ulnar nerve. On day 10, a prominent conduction block (CB) was seen after stimulation above and below the elbow without TD. On day 20, after the proximal CMAP amplitude had increased, the CB had disappeared. (C) RCF in intermediate and distal nerve segments of the ulnar nerve. The CB across the elbow had resolved rapidly and the proximal CMAP amplitude was greatly increased on day 11. (D) Improvement of RCF in the distal segment of the median nerves reveals abnormal amplitude reduction in the intermediate segment on day 25 (reproduced from Uncini et al. [27] with the permission of Elsevier).
Gangliosides are major target antigens of axonal GBS. The complement system is activated when immunoglobulin-G antibodies bind to gangliosides located in a node of Ranvier. Axonal degeneration proceeds by forming a membrane attack complex on the axolemma of motor fibers.24 Because AMCBN shares the same pathophysiology as AMAN, a rapid recovery of disrupted nodes in the above process is considered to be AMCBN.8
These AMCBN patients could be misclassified as demyelinating neuropathy by applying Hadden criteria at the initial NCS. Various new electrophysiological criteria have been proposed in attempts to overcome this limitation.
NEWLY SUGGESTED ELECTRODIAGNOSTIC CRITERIA
Rajabally and colleagues applied stricter values of DML, motor CV, and F-wave latencies in their diagnostic criteria of AIDP (Table 1).25 If there were no demyelinating features, the absence of F-waves and a proximal-to-distal CMAP amplitude ratio of <0.7 were considered the axonal features of GBS.
While the Rajabally criteria are highly sensitive for diagnosing axonal GBS, it remains difficult to diagnose AIDP.26,27 Uncini and colleagues proposed taking the findings of the second NCS into consideration (Table 2).26,27 Patients with continuously low CMAP amplitudes were categorized into an axonal degeneration group, and the following criteria were applied to the follow-up study: the distal CMAP amplitudes increasing by more than 150% without an increased distal CMAP duration (distal RCF), or the RCF recovering due to the proximal CMAP amplitude increasing (Fig. 1). Applying these revised criteria significantly reduced the overall error rate to 30.0% compared to those for the Hadden criteria (48.0%) and the Rajabally criteria (45.0%).27,28
CONCLUSION
Electrophysiological findings in GBS play an important role not only in its diagnosis but also in understanding the pathophysiology via the discovery of CB. It is necessary to conduct serial NCS studies because the results change markedly during the time course of the disease. If only one study is to be performed, an accurate diagnosis will require the application of as many criteria as possible.
Acknowledgements
This work was partly supported by Dong-A University Hospital and a National Research Foundation of Korea (NRF) grant funded by the Korean Ministry of Education, Science and Technology (MEST) (grant no. 2016R1A5A2007009). This work was also partly supported by the Basic Science Research Program through the NRF funded by the MEST (grant no. NRF-2017R1D1A1B03029672).
Notes
The authors declare no conflicts of interest relevant to this article.