
|
| About the Journal |
| Aims and Scope |
| Journal Information |
| Editorial Board |
| Best Practice |
| Subscriptions |
| Contact Us |


|
| About the Journal |
| Aims and Scope |
| Journal Information |
| Editorial Board |
| Best Practice |
| Subscriptions |
| Contact Us |

AbstractVestibular dysfunction has rarely been reported in MELAS syndrome. A 40-year-old male with long-term diabetes and hearing loss experienced a stroke-like episode with hemisensory disturbance and lactic acidosis. Brain MRI showed temporo-parieto-occipital cortical lesions, and a final diagnosis was made of MELAS syndrome with the mitochondrial 3243A>G mutation. Neuro-otologic evaluations revealed anterior-canal-sparing bilateral impairments of the vestibulo-ocular reflex in the video head impulse test and no caloric paresis. This unique pattern of vestibular dysfunction may aid in diagnosing MELAS syndrome.
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is a rare, maternally inherited mitochondrial disorder that usually occurs in association with the mitochondrial 3243A>G mutation in the MT-TL1 gene encoding tRNA.1 MELAS syndrome may show multiorgan involvement, which results in various clinical manifestations that include dementia, epilepsy, diabetes mellitus (DM), and short stature.1 The neuro-otologic complications are mild but insidiously progressive. Sensorineural hearing loss commonly occurs in 71-77% of individuals with MELAS syndrome, whereas vertigo or vestibular dysfunction has rarely been reported.1 Here we report a case of MELAS syndrome presenting with a distinguishing pattern of bilateral vestibulopathy with bilateral hearing loss and ocular motor abnormalities, and review the relevant literature.
CASEA 40-year-old male presented with paresthesia of the left hemibody with a 1-month history. He also complained of dizziness with progressive imbalance for several months. He had a 10-year history of type-2 DM and a 7-year history of progressive bilateral hearing loss. He denied any history of treatment with potentially ototoxic drugs, including antibiotics. His mother and elder sister both have a history of early-onset DM with hearing loss. Neurologic examinations revealed a loss of all sensory modalities in his left hemibody, bilateral dysmetria, and truncal ataxia. Confrontation visual field testing revealed left homonymous hemianopsia. Other neurologic evaluations, including motor function and pathologic reflex, produced unremarkable findings. In brain magnetic resonance imaging (MRI), T2-weighted fluid-attenuated inversion recovery images showed cortical and subcortical hyperintense lesions with gyral swelling in the right cerebral hemisphere. These lesions were also detected in other MRI sequences, including diffusion-weighted images (Fig. 1A-D). However, brain MR angiography revealed no relevant intracerebral arterial stenosis with the corresponding lesions (Fig. 1E). It was particularly notable that diffuse cerebellar atrophy was also evident (Fig. 1F). Laboratory evaluations showed elevations of serum lactate at 4.1 mmol/L (normal range, 0.5-2.2 mmol/L) and HbA1c at 8.3% (normal range, 4.4-6.4%). A tentative diagnosis of MELAS syndrome was made based on the clinical presentation, neuroradiologic findings, and lactic acidosis. The mitochondrial 3243A>G mutation in MT-TL1 was finally detected, which confirmed the diagnosis.
We evaluated the patient’s dizziness with progressive imbalance by performing laboratory neuro-ophthalmologic and neuro-otologic investigations (Table 1). Video-oculography revealed horizontal gaze-evoked nystagmus (GEN), bilaterally impaired smooth pursuit, and hypometric saccades. The bedside horizontal head impulse test (HIT) showed abnormal catch-up saccades (CS) bilaterally, and dynamic visual acuity decreased markedly. Moreover, the video HIT, which is a novel method for objectively measuring the vestibulo-ocular reflex (VOR), documented bilaterally decreased VOR gains with abnormal CS for the horizontal and posterior canals, but normal VOR function in the anterior canals (ACs) (Fig. 2A). However, bithermal caloric test results and vestibular-evoked myogenic potentials (VEMPs; both cervical and ocular) were normal. Pure-tone audiometry showed moderately severe sensorineural hearing loss bilaterally (Fig. 2B).
DISCUSSIONThe mitochondrial 3243A>G mutation produces diverse phenotypes according to the organs involved along with various combinations of symptoms, including maternally inherited deafness and diabetes (MIDD), progressive external ophthalmoplegia, and MELAS syndrome in severe cases.1 Despite the milder clinical manifestations and the insidious nature of MIDD syndrome compared with MELAS syndrome, both syndromes may be associated with various degrees of vestibular dysfunction as well as hearing loss.2 However, the frequency and underlying pathomechanisms of vestibular dysfunction associated with the mitochondrial 3243A>G mutation are still uncertain.3
A previous cohort study of the prevalence of vestibular dysfunction in adults with mitochondrial disease found that 40 (35%) of 114 patients had a suspected balance disorder, and that 30 (91%) of 33 patients exhibited vestibular dysfunction in neuro-otologic investigations.3 Sixteen (53.3%) of 30 patients with vestibular dysfunction showed the mitochondrial 3243A>G mutation (one with MELAS syndrome and 11 with MIDD syndrome), and 12 (75%) of them showed unilateral (n = 7) or bilateral (n = 5) vestibulopathy.3 Those authors concluded that vestibular dysfunction is not an infrequent manifestation in mitochondrial disease in adults and so should be evaluated. Another study also showed various degrees of vestibular dysfunction in 12 (92.3%) of 13 patients with the mitochondrial 3243A>G mutation, as assessed by a caloric test and VEMPs (six with MELAS syndrome and six with MIDD syndrome).4
The mitochondrial 3243A>G mutation may produce signs of cerebellar dysfunction including dysmetria, truncal ataxia, and cerebellar atrophy in neuroimaging.5 Moreover, central ocular motor signs such as saccadic dysmetria, impaired smooth pursuit, and horizontal GEN can also be observed.2,5 A previous study suggested that cerebellar (especially flocculus) dysfunction can contribute to vestibular abnormalities associated with the mitochondrial 3243A>G mutation, based on the selective AC-sparing VOR impairments in video HITs (high-frequency stimuli) without canal paresis in the caloric test (low-frequency stimuli), as well as central ocular motor signs and cerebellar atrophy in brain MRI.5 The clinical presentation of our patient was consistent with that previous study.5 Indeed, similar patterns of VOR impairments in patients with the mitochondrial 3243A>G mutation have been reported previously (Table 1).2,5,6 Experimental studies have demonstrated that the reduced VOR gains with high-frequency stimuli might be attributable to frequency-dependent VOR enhancement in the flocculus.7 Moreover, the loss of inhibitory projections of the flocculus to the AC pathway may result in a higher VOR gain for the AC during downward HITs.7 Taking these results together with the clinical and neuroradiologic findings suggesting cerebellar involvement in our patient, it seems plausible that cerebellar dysfunction plays a role in the vestibular dysfunction associated with the mitochondrial 3243A>G mutation. Conversely, the AC-sparing VOR impairments in video HITs in bilateral vestibulopathy can also be observed in association with peripheral vestibular damage such as aminoglycoside-related vestibulotoxicity or Ménière’s disease, which is potentially related to reduced vulnerability or superior recovery of the ACs.8 However, the case series in Table 1, including our case, mostly showed normal caloric responses (4/4, 100%) and abnormal pursuit eye movements (5/5, 100%). Thus, these findings support that the cerebellum may be responsible for the unique patterns of bilateral vestibulopathy with ocular motor dysfunction associated with the mitochondrial 3243A>G mutation.
In summary, we observed a patient with MELAS syndrome with AC-sparing bilateral vestibulopathy. Vestibular dysfunction is not an infrequent manifestation of the mitochondrial 3243A>G mutation,2-5,9 which makes detailed neuro-otologic evaluations crucial for these patients who experience dizziness or imbalance. Moreover, the distinctive pattern of their vestibular impairments may aid in the differential diagnosis of bilateral vestibulopathy, although evidence from large samples is still required.
AcknowledgementsThis study was performed in accordance with the recommendations of the Institutional Review Board of the Chonnam National University Hospital (Gwangju, South Korea).
Fig. 1.In brain magnetic resonance imaging, a T2-weighted fluid-attenuated inversion recovery image (A) shows cortical and subcortical hyperintense lesions with gyral swelling in the right temporo-parieto-occipital and insular lobes, which could not be defined as the involvement of specific arteries (white arrows). The corresponding lesions were associated with diffusion restriction in diffusion-weighted images, hypo-/iso-/hyperintensities in an apparent diffusion coefficient sequence, and gyral enhancement in gadolinium-enhanced T1-weighted images (B-D, dotted arrows). Magnetic resonance angiography (E) reveals no relevant intracerebral arterial stenosis. A sagittal T1-weighted image (F) shows diffuse cerebellar atrophy without definite brainstem involvement. 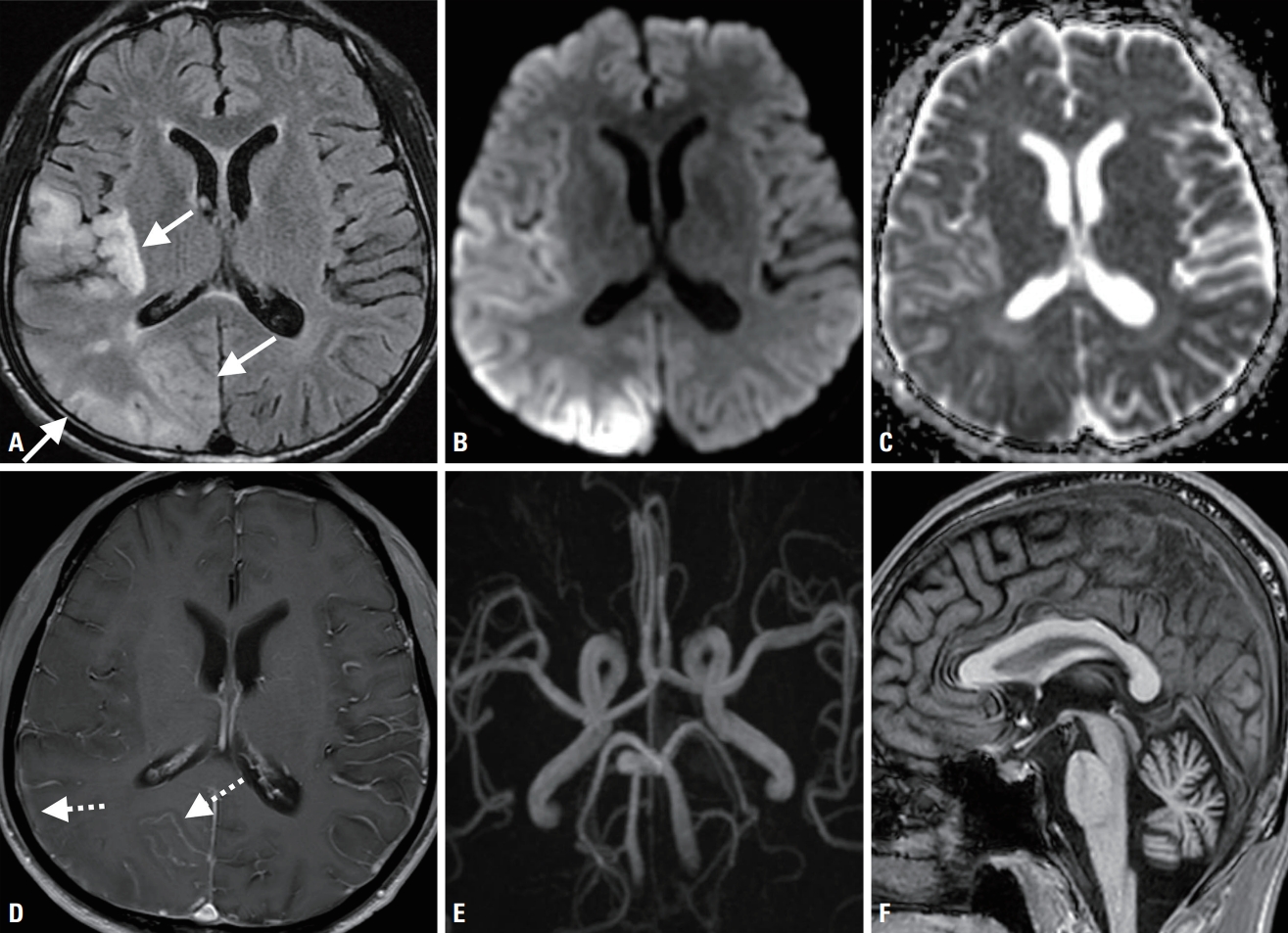
Fig. 2.The video head impulse test (A) revealed decreased gains of the vestibulo-ocular reflex (VOR) in bilateral horizontal and posterior canals (HCs and PCs, respectively) combined with abnormal catch-up saccades, without anterior canal (AC) impairment (red traces). Pure-tone audiometry (B) demonstrated moderately severe sensorineural hearing loss bilaterally, by about 60 and 70 dB in the right and left ears, respectively. Note: decreased VOR gains were defined as < 0.8 and < 0.7 for the HCs and vertical canals, respectively. 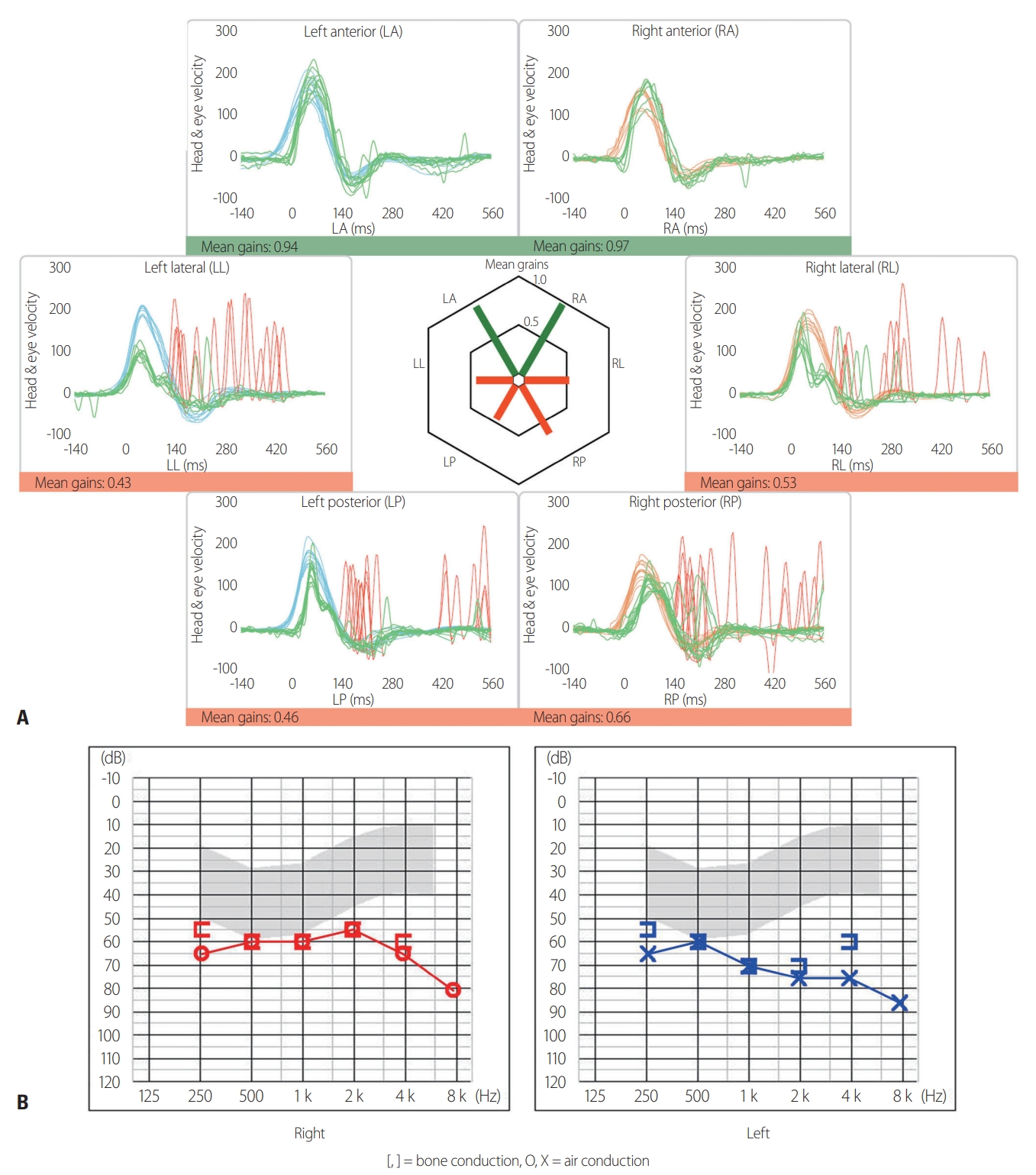
Table 1.Video-oculography and neuro-otologic findings of patients with the mitochondrial 3243A>G mutation in MT-TL1
Vestibulo-ocular reflex (VOR) gains were measured using the video head impulse test (ICS impulse; GN Otometrics, Taastrup, Denmark) in five subjects (cases 1, 5-8), and decreased VOR gains were defined as < 0.8 and < 0.7 for the horizontal and vertical canals, respectively. RA, right anterior canal; LA, left anterior canal; RH, right horizontal canal; LH, left horizontal canal; RP, right posterior canal; LP, left posterior canal; R, right; L, left; M, male; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes; GEN, gaze-evoked nystagmus; F, female; SWJ, square-wave jerk; HN, horizontal nystagmus; UBN, upbeat nystagmus; SN, spontaneous nystagmus; CCW, counterclockwise; TN, torsional nystagmus; MIDD, maternally inherited deafness and diabetes; N/A, not applicable; SD, standard deviation. a In three patients from Kim et al. 5 (cases 2-4), eye-movement abnormalities were assessed repeatedly in two subjects (cases 3 and 4), and the normal range of VOR gains differed from the other cases since they were tested using a magnetic search-coil technique. REFERENCES1. El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab 2015;116:4-12.
2. Cardenas-Robledo S, Saber Tehrani A, Blume G, Kattah JC. Visual, ocular motor, and cochleo-vestibular loss in patients with heteroplasmic, maternally-inherited diabetes mellitus and deafness (MIDD), 3243 transfer RNA mutation. J Neuroophthalmol 2016;36:134-140.
3. Holmes S, Male AJ, Ramdharry G, Woodward C, James N, Skorupinska I, et al. Vestibular dysfunction: a frequent problem for adults with mitochondrial disease. J Neurol Neurosurg Psychiatry 2019;90:838-841.
4. Iwasaki S, Egami N, Fujimoto C, Chihara Y, Ushio M, Kashio A, et al. The mitochondrial A3243G mutation involves the peripheral vestibule as well as the cochlea. Laryngoscope 2011;121:1821-1824.
5. Kim SH, Akbarkhodjaeva ZA, Jung I, Kim JS. Eye movement and vestibular dysfunction in mitochondrial A3243G mutation. Neurol Sci 2016;37:1159-1162.
6. Kattah JC. Clinical characteristics and etiology of bilateral vestibular loss in a cohort from central illinois. Front Neurol 2018;9:46.
7. Walker MF, Zee DS. Asymmetry of the pitch vestibulo-ocular reflex in patients with cerebellar disease. Ann N Y Acad Sci 2005;1039:349-358.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||