Increased intracranial pressure (IICP) due to a posterior fossa tumor can lead to a hydrocephalic fit with loss of consciousness. The recent development of brain imaging modalities has enabled the earlier diagnosis and management of brain tumors,1,2 and diagnosis of brain tumors at a younger age has led to a decline in the incidence of hydrocephalic fits. Hydrocephalus usually progresses slowly and is rarely the cause of seizures.3
Tectal glioma arises in the tectum of the midbrain and causes aqueductal stenosis due to its anatomical location, which leads to IICP. Tectal glioma occurs predominantly in the pediatric population and typically presents with IICP signs such as headache, vomiting, and papilledema. It occurs only rarely in adults, comprising only 1% of all brain tumors in that population.4,5
Here we report a patient who presented with adult-onset focal impaired awareness seizure caused by obstructive hydrocephalus associated with a tectal glioma. We describe in detail the patient’s diagnosis and treatment process, and discuss future treatment plans.
CASE
A 34-year-old, right-handed female presented with recurrent loss of consciousness and anxiety. She had experienced sudden attacks of fear once or twice a month starting 2 years before she visited the hospital. Although witnesses were not present because the patient was a homemaker and raised her child alone during the day, she reported loss of consciousness that occurred approximately two or three times a week. Immediately before the symptoms, she experienced palpitations and vertigo followed by déjà vu. At the onset of symptoms, she was under severe stress due to years of divorce litigation. One year before hospitalization, episodes of palpitations and vertigo occurred approximately five or six times almost every day, which had led to her visiting a psychiatric clinic.
The patient was diagnosed with depression and panic disorder, and was prescribed escitalopram (10 mg/day), alprazolam (0.5 mg/day), and clonazepam (0.5 mg/day); however, her symptoms did not improve. One week before she presented to the hospital, she experienced a witnessed bilateral tonic-clonic seizure with turning of the head to the left and shouting during sleep. She had never received brain imaging prior to this hospital visit. The patient had undergone total thyroidectomy for thyroid cancer at 26 years of age and was taking a thyroid hormone replacement. There were no other underlying diseases.
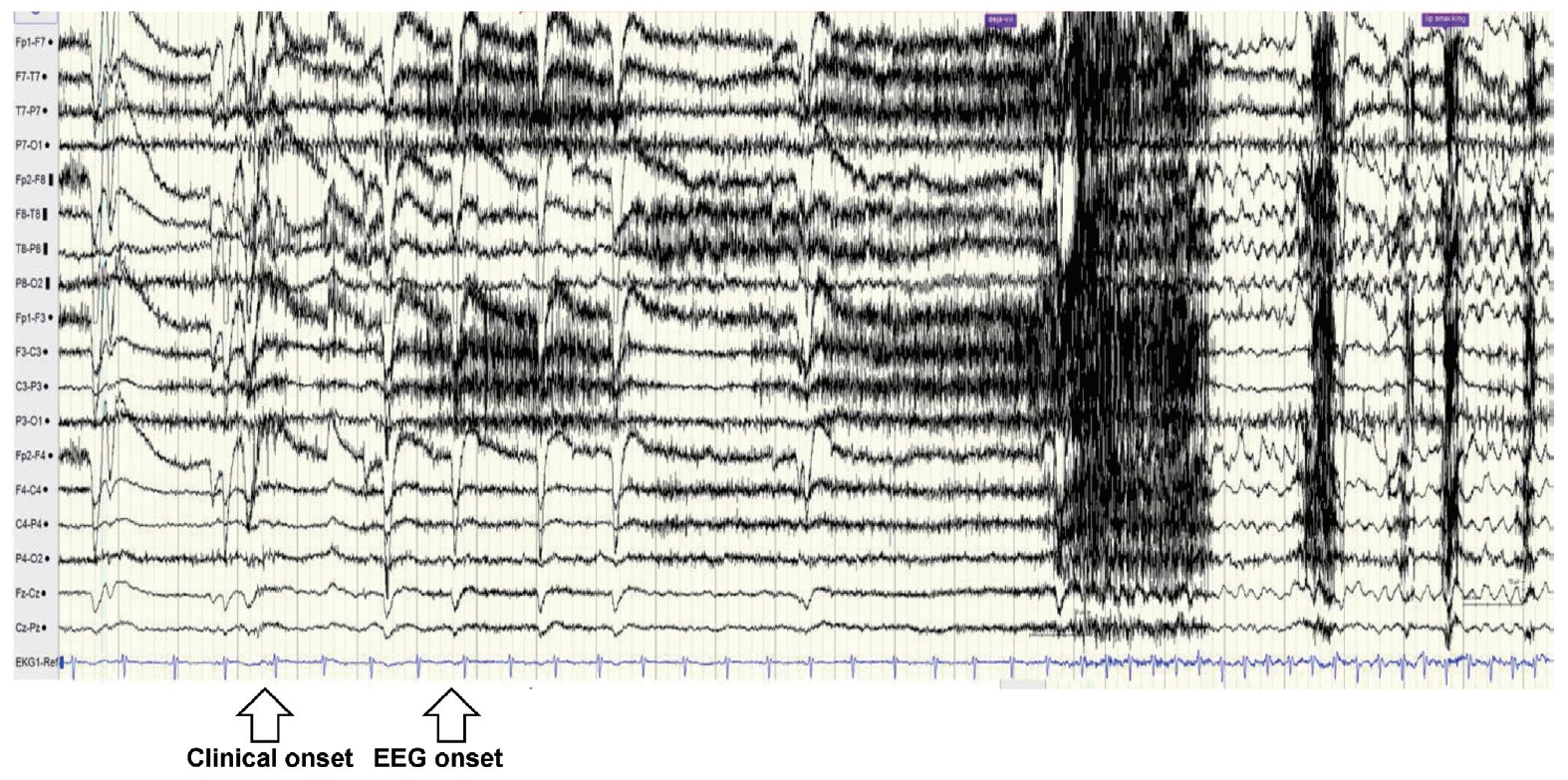
The patient was admitted to the video-electroencephalogram (EEG) monitoring unit to determine whether her frequent episodes of palpitations and vertigo were epileptic seizures. The neurological examination did not show any abnormal findings, and no subjective symptoms other than mild anxiety were observed. Two seizure attacks were identified during the 24-hour monitoring period. The first attack was a focal impaired awareness seizure that progressed to automotor seizure after déjà vu. The second attack was also a focal impaired-awareness seizure leading to automotor seizure after experiencing palpitations and vertigo. The patient remembered only the aura in both seizures and had not been aware of the automatism.
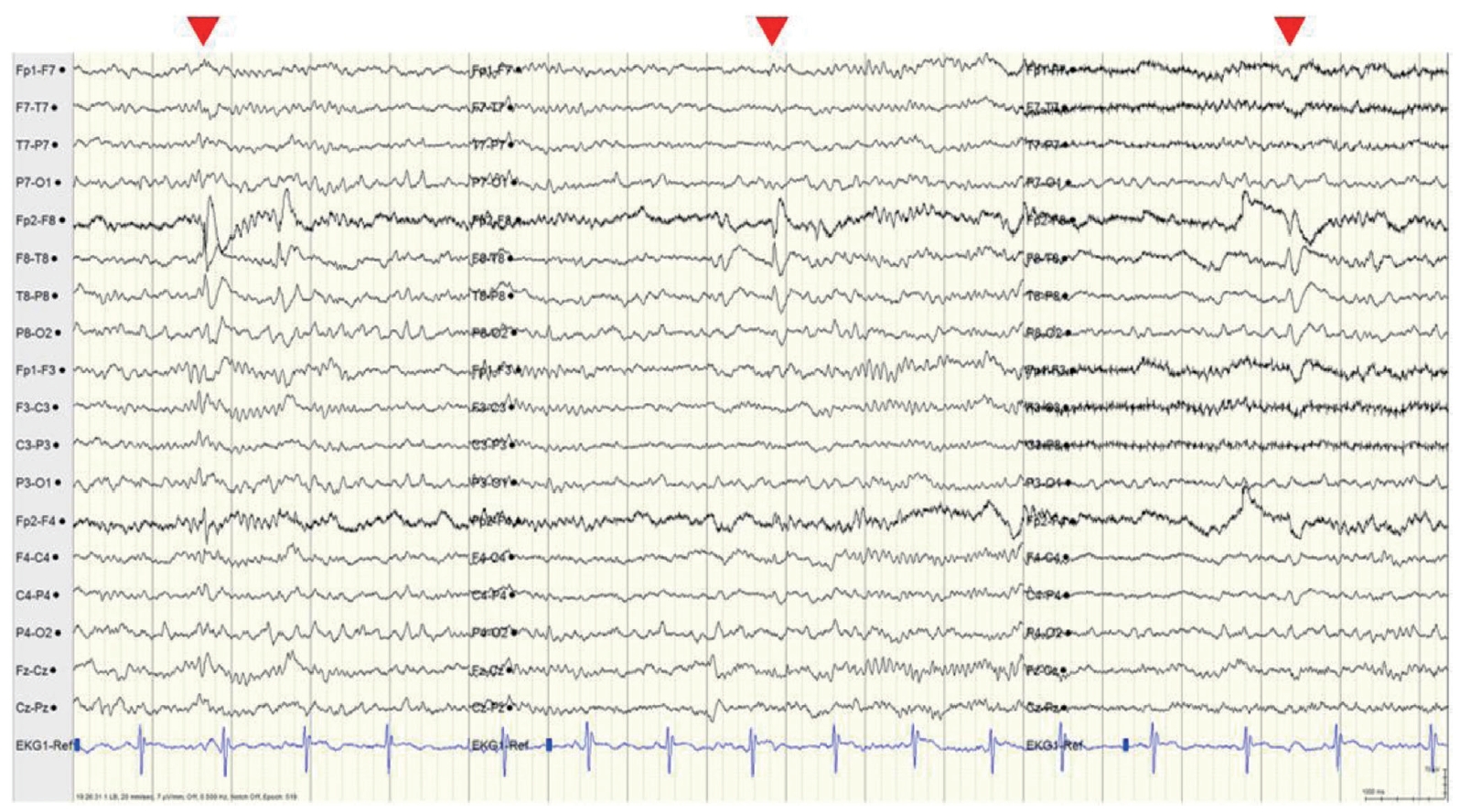
Intermittent slowing was observed in both temporal areas on the interictal EEG, especially in the right temporal area, and epileptiform discharges were recorded in the right anterior temporal area (F8 or F8-T8) at a frequency of once every 5-10 seconds during sleep (Fig. 1). The ictal EEG began in the right anterior temporal area and spread to the right hemisphere during both events. Ictal tachycardia was observed; her preictal heart rate was about 75 beats/min, rising during the ictal event to 150 beats/min (Fig. 2).
Brain magnetic resonance imaging (MRI) performed immediately before the video-EEG monitoring test revealed a 0.7-cm-diameter tumor in the periaqueductal gray area. Based on neuroradiological reading, tectal glioma was the most likely diagnosis; obstructive hydrocephalus was accompanied by aqueductal stenosis. On T2-weighted and T2-weighted fluid-attenuated inversion recovery images, high signal intensity and mild atrophy were observed in both anterior temporal areas, which were considered to be either seizure-related changes or cortical dysplasia (Fig. 3A).
Based on the clinical manifestation, EEG, and MRI findings, a diagnosis of right anterior temporal lobe epilepsy was possible. Symptoms or signs of IICP were not observed, thus eliminating the possibility of secondary injury of temporal area and associated epileptic seizure caused by obstructive hydrocephalus due to tectal glioma was difficult. Therefore, endoscopic third ventriculostomy was performed to control the hydrocephalus. In addition, antiepileptic drugs (oxcarbazepine at 300 mg/day and clobazam at 10 mg/day) were administered to control frequent aura and focal impaired awareness seizures.
The anxiety, palpitations, and vertigo of the patient improved immediately after surgery, and the focal impaired awareness seizures that had occurred several times every day prior to surgery had completely resolved. Three-month postoperative MRI revealed that the size of the ventricles and high signal intensities in both anterior temporal areas were slightly decreased compared with the preoperative imaging (Fig. 3B). On follow-up video-EEG monitoring performed at the same time, the rate of epileptiform discharges observed in the right anterior temporal area (F8 or F8-T8) had decreased to once per minute during sleep, but still persisted. Seizures did not occur during sleep. There was no significant improvement on brain MRI performed 8 months after the surgery (Fig. 3C); however, the EEG had normalized. The patient did not report complications related to surgery or side effects from taking antiepileptic drugs.
DISCUSSION
Tectal glioma is a glioma that arises in the midbrain and is rare in adults.4 It is a benign tumor that occurs mainly in children. A high seizure prevalence rate of 12.1% (8 of 66) has been reported; however, 50% were seizures not related to the tumor, such as febrile seizure, meningitis, and postoperative sequelae, and tectal glioma was discovered incidentally during the seizure evaluation in 25% of the patients.3 In addition, all eight patients with reported seizures had symptoms before the age of 11 years.
The prevalence of epileptic seizures in adult patients with tectal glioma is extremely low and has been studied previously, mainly because most cases are detected in children and treated early.6 To the best of our knowledge, this is the first reported case of epileptic seizures in a patient with tectal glioma that was not detected until adulthood. Due to its location, tectal glioma generally presents with IICP symptoms such as headache, nausea, and visual disturbances. Therefore, conservative management to improve symptoms by controlling intracranial pressure is prioritized.4,7 The tumor location is difficult to approach surgically and the course of the disease is mostly benign, and so the glioma is usually closely monitored using follow-up imaging. Historically, a ventriculoperitoneal shunt was placed to control IICP, but complications such as repetitive obstruction and shunt infection rendered this procedure problematic. Endoscopic third ventriculostomy has recently become recognized as the standard treatment.4,8
In the present case, although a considerable degree of obstructive hydrocephalus was present due to the tectal glioma, the patient had reached adulthood without any signs of IICP. Whether her right anterior temporal lobe epilepsy was secondary to brain injury caused by hydrocephalus or was a primary occurrence was unclear. In addition, due to the accompanying anxiety disorder, which is similar to the aura in temporal lobe epilepsy, the diagnosis of epilepsy was delayed for more than 2 years after symptom onset. In most cases of hydrocephalic fits caused by tectal glioma in children, clinical seizures are improved by decompression alone and antiepileptic drugs are not administered. However, as in the present case, when tectal glioma is first detected as an epileptic seizure in an adult, it is necessary to determine whether it is an epileptic seizure associated with secondary brain injury due to long-term hydrocephalus rather than a hydrocephalic fit, and perform only decompression surgery.
In Korea, a brain tumor accompanying hydrocephalus is rarely missed because brain imaging is performed preferentially when a patient with generalized seizure or evident complex partial seizure visits a clinic. However, if consciousness is partially preserved, such as in nondominant temporal lobe epilepsy, or if the aura is similar to symptoms of anxiety disorder or panic disorder, accurate diagnosis and treatment may be delayed.9
There is a dearth of studies on the course and prognosis of tectal glioma in adults due to its rarity in that population. Moreover, there are no guidelines on how long to continue antiepileptic drug administration if clinical seizures improve after decompression surgery but the atrophy and high signal intensity in the temporal area persist in follow-up imaging, or if epileptiform discharges persist in that region on the EEG. The authors plan to perform brain imaging and EEG measurements on a regular basis in this patient to monitor for any changes while she continues to take the same dose of antiepileptic drugs. We hope this case can serve as a basis for studies of the pathophysiology and prognosis of epilepsy associated with adult tectal glioma.